PIB Headquarters
PIB’S BULLETIN ON COVID-19
Posted On:
27 JAN 2022 6:00PM by PIB Delhi


- Over 164 cr vaccine doses have been administered so far under Nationwide Vaccination Drive
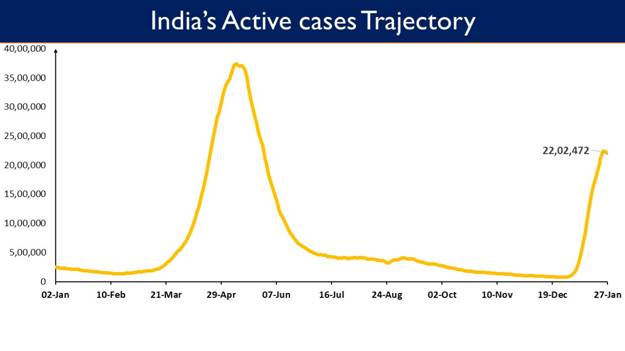
- India's Active caseload currently stands at 22,02,472
- Active cases stand at 5.46%
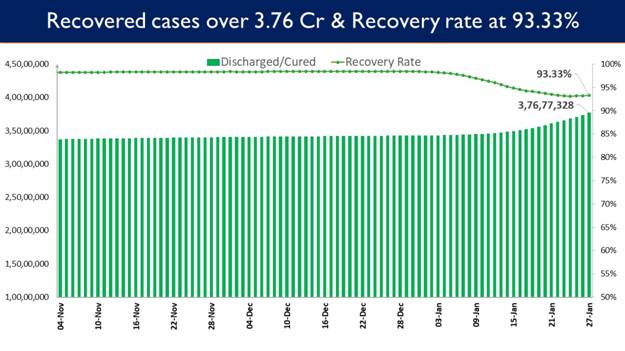
- Recovery Rate currently at 93.33%
- 3,06,357 recoveries in the last 24 hours increases Total Recoveries to 3,76,77,328
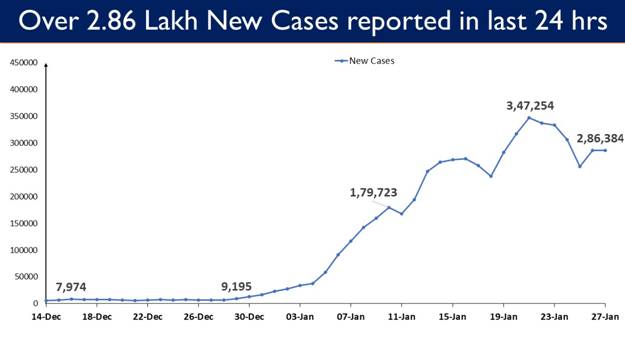
- 2,86,384 new cases recorded in the last 24 hours
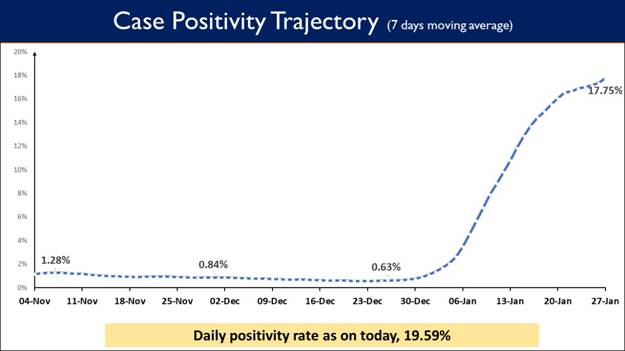
- Daily positivity rate (19.59%)
- Weekly Positivity Rate (17.75%)
- 72.21 cr Total Tests conducted so far; 14,62,261 tests conducted in the last 24 hours
|
#Unite2FightCorona #IndiaFightsCorona
PRESS INFORMATION BUREAU
MINISTRY OF INFORMATION & BROADCASTING
GOVERNMENT OF INDIA
*****
India’s Cumulative COVID-19 Vaccination Coverage exceeds 164 Cr
More than 22 lakh Vaccine Doses administered in the last 24 hours
Recovery Rate currently stands at 93.33%
2,86,384 New Cases reported in the last 24 hours
India's Active Caseload currently stands at 22,02,472
Weekly Positivity Rate is presently at 17.75%
India’s Cumulative COVID-19 Vaccination Coverage has exceeded 164 Cr today afternoon.
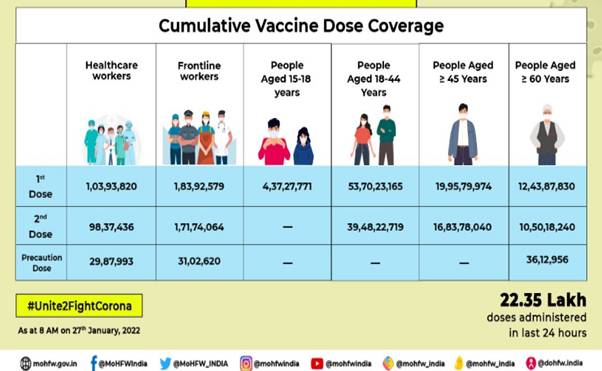
This has been achieved through 1,78,47,482 sessions. The break-up of the cumulative figure as per the provisional report till 7 am today include:
|
Cumulative Vaccine Dose Coverage
|
|
HCWs
|
1st Dose
|
1,03,93,820
|
|
2nd Dose
|
98,37,436
|
|
Precaution Dose
|
29,87,993
|
|
FLWs
|
1st Dose
|
1,83,92,579
|
|
2nd Dose
|
1,71,74,064
|
|
Precaution Dose
|
31,02,620
|
|
Age Group 15-18 years
|
1st Dose
|
4,37,27,771
|
|
Age Group 18-44 years
|
1st Dose
|
53,70,23,165
|
|
2nd Dose
|
39,48,22,719
|
|
Age Group 45-59 years
|
1st Dose
|
19,95,79,974
|
|
2nd Dose
|
16,83,78,040
|
|
Over 60 years
|
1st Dose
|
12,43,87,830
|
|
2nd Dose
|
10,50,18,240
|
|
Precaution Dose
|
36,12,956
|
|
Precaution Dose
|
97,03,569
|
|
Total
|
1,63,84,39,207
|
3,06,357 patients have recovered in the last 24 hours and the cumulative tally of recovered patients (since the beginning of the pandemic) is now at 3,76,77,328.
Consequently, India’s recovery rate stands at 93.33%.
2,86,384 new cases were reported in the last 24 hours.
India’s Active Case load is presently at 22,02,472. Active cases constitute 5.46% of the country's total Positive Cases.
The testing capacity across the country continues to be expanded. The last 24 hours saw a total of 14,62,261 tests being conducted. India has so far conducted over 72.21 Cr (72,21,66,248) cumulative tests.
While testing capacity has been enhanced across the country, Weekly Positivity Rate in the country currently stands at 17.75% and the Daily Positivity rate also reported to be 19.59%.
https://www.pib.gov.in/PressReleasePage.aspx?PRID=1792888
Update on COVID-19 Vaccine Availability in States/UTs
More than 163.71 Crore vaccine doses provided to States/UTs
More than 13.60 Crore balance and unutilized vaccine doses still available with States/UTs
The Union Government is committed to accelerating the pace and expanding the scope of COVID-19 vaccination throughout the country. The nationwide COVID 19 vaccination started on 16th January 2021. The new phase of universalization of COVID-19 vaccination commenced from 21st June 2021. The vaccination drive has been ramped up through availability of more vaccines, advance visibility of vaccine availability to States and UTs for enabling better planning by them, and streamlining the vaccine supply chain.
As part of the nationwide vaccination drive, Government of India has been supporting the States and UTs by providing them COVID Vaccines free of cost. In the new phase of the universalization of the COVID19 vaccination drive, the Union Government will procure and supply (free of cost) 75% of the vaccines being produced by the vaccine manufacturers in the country to States and UTs/
|
VACCINE DOSES
|
(As on 27th January, 2022)
|
|
SUPPLIED
|
1,62,73,06,725
|
|
BALANCE AVAILABLE
|
13,83,03,116
|
More than 163.71 crore (1,63,71,18,725) vaccine doses have been provided to States/UTs so far through Govt. of India (free of cost channel) and through direct state procurement category.
More than 13.60 Cr (13,60,98,246) balance and unutilized COVID Vaccine doses are still available with the States/UTs to be administered.
https://www.pib.gov.in/PressReleasePage.aspx?PRID=1792884
National Regulator approves “Conditional Market Authorization” of two COVID19 Vaccines- Covaxin and Covishield
Market Authorization conditional to submission of ongoing clinical trial data and safety data of the vaccine, at longer time intervals
All vaccinations to be recorded on CoWIN platform and AEFI, AESI to be strictly monitored
The National Regulator, Drugs Controller General of India (DCGI), has given nod to market authorization of two COVID19 vaccines, Covaxin and Covishield subject to certain conditions, here today. The Subject Expert Committee (SEC) of the Central Drugs Standard Control Organization (CDSCO) had recommended for upgradation of status for the vaccines from restricted use in emergency situations to grant of new drug permission with conditions in the adult population on 19th January 2022.
The proactive and agile approach followed by Government of India has been a hallmark of its strategy of management of COVID19. The latest approval accorded by DCGI for conditional market authorization to two COVID19 vaccines in the country indicates the promptness and timeliness with which the public response strategy and decision making apparatus of the country has responded to the emerging needs during the pandemic.
It may be noted that of the global Stringent Regulatory Authorities, only the United States Food and Drug Administration (USFDA) Medicines and Healthcare products Regulatory Agency (MHRA) of the UK have granted “conditional market authorization” to Pfizer and AstraZeneca, respectively, for their COVID19 Vaccines.
https://www.pib.gov.in/PressReleasePage.aspx?PRID=1792956
Tweet Links:
******
AS
(Release ID: 1792998)
Visitor Counter : 840