Ministry of Science & Technology
Dr Harsh Vardhan launches CSIR partnered clinical trials website “CUReD” on Repurposed Drugs for Covid- 19
Website provides information about Covid-19 drugs, diagnostics and devices including current stage of trials
CSIR is also working with Ministry of AYUSH for clinical trials of AYUSH drugs including AYUSH-64
Dr Harsh Vardhan emphasizes on maintaining social distancing, wearing masks and other precautions as essential in the fight against COVID-19
Posted On:
20 OCT 2020 6:50PM by PIB Delhi
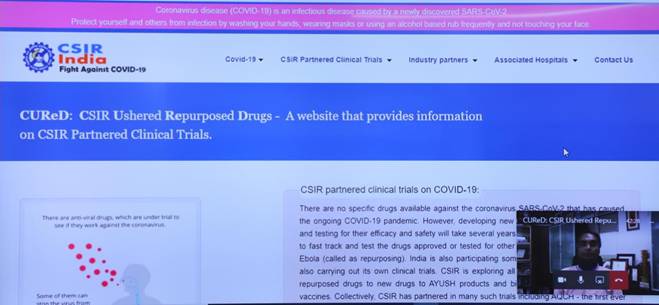
Dr Harsh Vardhan, Minister of Science & Technology, Health and Family Welfare and Earth Sciences, today launched a website that gives comprehensive information about the numerous COVID-19 clinical trials that CSIR is engaged in partnership with Industry, other government departments and ministries.
Called CuRED or CSIR Ushered Repurposed Drugs, the website provides information about the drugs, diagnostics and devices including the current stage of the trials, partnering institutions and their role in the trials and other details. The site can be accessed at https://www.iiim.res.in/cured/ or
http://db.iiim.res.in/ct/index.php.


The Minister lauded the efforts of CSIR for being at the forefront of the ongoing fight against COVID-19 and prioritizing clinical trials, generating data for their regulatory approval and helping launch drugs and diagnostics in the market. He commended the approach of using repurposed drugs and also, synthesizing COVID-19 drugs through new processes and transferring to Industry.
CSIR is exploring multiple combination clinical trials of anti-virals with host-directed therapies for the potential treatment of COVID-19. CSIR is also working with the Ministry of AYUSH for clinical trials of AYUSH drugs and has undertaken safety & efficacy trials of AYUSH prophylactics and therapeutics based on individual plant-based compounds and in combination. Five clinical trials involving Withaniasomnifera, Tinosporacordifolia + Piper longum(in combination), Glycyrrhizaglabra, Tinosporacordifolia & Adhatodavasica (individually and in combination) and AYUSH-64 formulation are undergoing safety and efficacy trials.
A key clinical trial of CSIR is the Sepsivac (Mw) against COVID -19 in partnership with Cadila. The phase 2 clinical trial has been completed successfully on critically ill COVID-19 patients, and more extensive Phase 3 trial is on the anvil. Further, the Phase 2 trial of phytopharmaceutical AQCH on Covid-19 patients with Sun Pharma and DBT is underway.
In addition to clinical trials of repurposed drugs and vaccines, CSIR has been involved in clinical trials of diagnostics and devices.
Dr Harsh Vardhan emphasized that while the scientists are working on developing drugs and vaccines, as of now, social distancing, wearing masks and other precautions are essential and must be duly followed if we have to win the fight against COVID-19.
Dr. Shekhar C Mande, Secretary, DSIR and DG-CSIR, Dr.Ranjana Aggarwal, Dir, NISTADS and Dr. Geetha Vani Rayasam, Senior Principal Scientist & Head, Science Communication and Dissemination Directorate CSIR HQ, were present on the occasion. CSIR Directors, Heads of Departments, and Scientists involved in Clinical Trials joined the event virtually.
*****
NB/KGS/(CSIR inputs)
(Release ID: 1666171)
Visitor Counter : 1303