Ministry of Science & Technology
Covaxin can help in controlling the virus load of SARS-CoV-2 & its variants, reducing disease severity: Study
Posted On:
15 JUL 2022 4:28PM by PIB Delhi
Scientists have found that Covaxin, which is an inactivated whole-virion vaccine, induces robust immune memory to SARS-CoV-2 and variants of concern that persist for at least 6 months after vaccination and induces memory T cells that can respond robustly against the variants. This may help in controlling the virus load and thus, reduce the disease severity.
BBV152/Covaxin vaccine is based on an Asp614Gly variant and formulated with a toll-like receptor (TLR) 7/8 agonist molecule (imidazoquinolin) adsorbed to alum. It was the first alum-imidazoquinolin adjuvanted vaccine produced in India and received emergency use authorization from WHO for use in a large population. Although the clinical trial data were available for the vaccine efficacy, important questions remained unanswered for the evidence-based policymaking particularly. These included whether the vaccine induces immune memory, how long the vaccine-induced memory persists, and whether these memory responses are able to sustain against the SARS-CoV-2 variants.
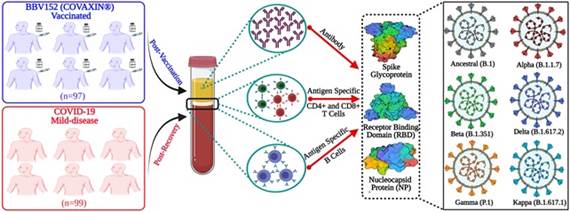
Scheme 1. The scheme representing the study design to determine the immunological effectiveness of the inactivated SARS-CoV-2 vaccine Covaxin®.
In a multi-institutional collaboration with THSTI, Faridabad, AIIMS, New Delhi, ESIC Medical College, Faridabad, LNJP Hospital, New Delhi, LJI, LA Jolla, Dr. Nimesh Gupta and group at the National Institute of Immunology (NII), New Delhi, investigated 97 SARS-CoV-2 unexposed individuals who had received vaccine, up to 6 months after 2-dose vaccination. The vaccine-induced responses were compared with the immune memory in 99 individuals recovered from mild COVID-19.
The study supported under IRHPA-COVID-19 special call by the Science and Engineering Research Board, a statutory body of the Department of Science and Technology, found that the vaccine produces antibodies against Spike, RBD, and Nucleoprotein of the virus, just like in virus infection. However, analyses of both the binding and neutralizing antibodies revealed a reduced recognition of variants of concern like Delta (India), Beta (S. Africa), and Alpha (UK).
This study showed that the vaccine is capable of inducing memory B cells. They found this satisfying because antibodies may decline with time, but these memory B cells can replenish antibodies against the virus, whenever required.
Their study provided the first-ever evidence of the detailed traits of immune memory generated in human in response to an inactivated virus vaccine.
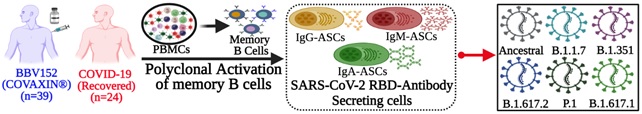
Scheme 2. The scheme represents the strategy to measure the SARS-CoV-2 specific memory B cells in the blood sample of participants received 2-dose Covaxin®.
The team also found that the vaccine showed potential of producing the SARS-CoV-2-specific T cells. Importantly, and unlike antibodies, the effectiveness of the T cells was well preserved against the variants. Also, these virus-specific T cells were present in the central memory compartment and persisted up to 6 months post-vaccination.
The SARS-CoV-2 variants may impact the antibody responses generated by vaccine; however, the T cell responses will be available to respond robustly against the variants. The study published in the Journal Nature Microbiology provides important knowledge for evidence-based policymaking on the future application of Covaxin®.
Publication link:
https://doi.org/10.1038/s41564-022-01161-5
*****
SNC / RR
(Release ID: 1841775)
Visitor Counter : 1745