|
Ministry of Science & Technology
Year End Review of Department of Biotechnology
DBT is one of the first Departments of Govt. of India to have successfully executed “Rationalization of Autonomous Bodies” by subsuming its 14 Autonomous Institutes under one APEX Body, Biotechnology Research and Innovation Council (BRIC) to enable process and performance enhancement India Bioeconomy Report 2023 launched by Union Minister S&T Notified Intellectual Property guidelines for maximizing socio-economic impact of research outcomes Major initiative on high-performance Biomanufacturing to foster integrated innovative research for development of bio-based products Biological Research Regulatory Approval Portal (BioRRAP) launched as a single gateway for regulatory approval for biological research Notified SoPs for regulatory review of Genome Edited Plants Developed ADVIKA, a superior drought tolerant high-yielding chickpea variety First gene therapy clinical trial in India for Hemophilia A Novel blood bag technology developed for decreasing damage of stored blood Development of India’s first Omicron booster mRNA vaccine Development of India’s 1st indigenous quadrivalent Human Papilloma Virus (qHPV) vaccine against cervical cancer Market Authorization Approval for Biosimilar Liraglutide for Type-2 Diabetes Manufacturing and commercialization approval for first-of-its kind indigenous MRI scanner developed under DBT’s National Biopharma Mission Organized Global Bio India (GBI) 2023 attended by 7000+ delegates from 500+ biotech startups, incubators, industry and academia 29 new biotech startup products launched in GBI
Posted On:
29 DEC 2023 7:37PM by PIB Delhi
The Department of Biotechnology (DBT), Government of India is at the forefront of promoting biotechnological innovation and entrepreneurship leveraging the strength of strategic partnerships and building capacities across the country. Over the years, DBT has created and nurtured a vibrant biotech research and innovation ecosystem in biotechnology and modern biology, in alignment with national missions such as AatmaNirbhar Bharat, Swasth Bharat, Swachh Bharat, Startup India and Make-in- India. All these efforts have resulted in a large number of patents and publications in the area of life sciences and biotechnology.
Major achievements of DBT during the year 2023 have been summarized below:
- Major Policy Reforms: The 14 Autonomous Institutions (AIs) of Department of Biotechnology (DBT) were subsumed under one Apex Autonomous Society viz. Biotechnology Research and Innovation Council (BRIC), for centralized and unified governance to maximize impact of biotech research across the country.
Subsequently, BRIC Society was registered in November 2023. This restructuring exercise aims to achieve both value and impact for strengthening & aligning science & technology (S&T) innovation ecosystem, to implement and enhance transformative power of S&T in defining bold global actions, to augment workforce transitions & capacity building; to enable immersion-based Ph.D. programme and to provide compelling incentives for harnessing frontier technologies and nurturing start-up ecosystem.
- Inculcation of Biotech Entrepreneurship Culture: The number of bioincubators increased to 75+ now, as compared to 6 in 2014. The number of Biotech Startups have increased to more than 6,000 in the last 9.5 years. From 10 biotech products in 2012, today this number has grown to over 800+ products. Nearly INR 4,500 Cr has been raised as follow on funding by 150 startups. Bioeconomy of the country has reached $137 billion in 2023 which is expected to reach $300 Bn by 2030.
- DBT IP Guidelines 2023: The Department, in consultation with various stakeholders, notified the DBT Intellectual Property (IP) Guidelines in September, 2023. These guidelines govern the transfer of IP from both extramural and intramural organizations funded by DBT. These guidelines have been framed to enable seamless transfer of IP at academic institutes/research laboratories towards commercialization into technologies/products for larger societal impact.
- New Initiative on Fostering High Performance Biomanufacturing: DBT has conceptualised a major initiative for fostering high-performance Biomanufacturing to promote innovation through discovery and integrated innovative research; bridge the gaps for scale-up; and promote manufacturing facilities for development of bio-based commercial products. This will be augmented through Bio-enabler Hubs such as Bio-Artificial Intelligence Hubs to support innovation and Biomanufacturing Hubs to promote scale up. This initiative is expected to facilitate growth of India’s Bioeconomy while promoting reduced Carbon-emission manufacturing processes.
- Launch of Biological Research Regulatory Approval Portal (BioRRAP): Keeping with the spirit of "One Nation, One Portal", the BioRRAP has been developed by DBT as a “whole of Government approach”. This portal is the 1st step in enabling ease of doing scientific research in India. The BioRRAP caters to all those seeking regulatory approval required for biological research and development activity in the country. This portal will strengthen interdepartmental synergies and bring accountability, transparency and efficacy in functioning of agencies regulating various aspects of biological research and issuing permission. The unique “BioRRAP ID” serves as a gateway to the portal and will help researcher to see stage of approval of the applications for regulatory clearances and also preliminary information on all the research work being undertaken by the particular researcher and/or organization.
- Standard Operating Procedures (SoPs) for regulatory review of Genome Edited Plants: DBT notified the SoPs for regulatory review of Genome Edited Plants under Site Directed Nuclease-1 (SDN-1) and SDN-2categories for enabling regulatory streamlining of genome edited plants and resilient crops for future. Plant genome editing is a promising technology in terms of applied biological research and innovation with a huge economic potential in a wide range of sectors. ‘Guidelines for the safety assessment of Genome Edited Plants’ were notified in May, 2022. Towards enabling biosafety regulation by Institutional Biosafety Committees (IBSCs), the SoPs and Checklist were drafted and notified to bring clarity to all the stakeholders.
- ADVIKA, a Superior Drought Tolerant High-Yielding Chickpea Variety: An improved drought tolerant desi chickpea variety “ADVIKA (NC 7)” was developed by introgression of an ABC transporter gene in the genetic background of JG 16 that enhances seed weight and yield (7% high) under drought stress. This superior drought tolerant chickpea variety is now approved by the Central Sub-committee on Crop Standards, Notification and Release of Varieties (CVRC), Ministry of Agriculture and Farmers Welfare, Government of India, for release and notification as a central variety for national use and cultivation, especially in the Central Zone of India.
- Accel Breed: a speed breeding facility at PAU, Ludhiana for accelerating crop improvement programme: Punjab Agricultural University (PAU) has developed a State-of-the-Art speed breeding facility, named “AccelBreed”. The facility contains eight environmentally controlled chambers where all the important environmental variables such as light, temperature, humidity and CO2 concentration required for the growth of the crop plants can be controlled at will.
- IRRI's SpeedFlower Protocol for Revolutionizing Rice Breeding for Global Food Security: International Rice Research Institute (IRRI) has established a state-of-the-art speed breeding facility at Varanasi, India and also developed novel SpeedFlower Protocol by smartly combining light parameters, such as a high red-to-blue spectrum ratio, with a 24-hour long day photoperiod, and optimizing growth conditions, including temperature and humidity. The SpeedFlower protocol not only expedites the development of new varieties but also contributes to addressing global food and nutritional security challenges.
- DBT’s Efforts for Vaccine Development: Significant advances have been witnessed in indigenous development of vaccines, as mentioned below:
- India’s 1st indigenously developed quadrivalent Human Papilloma Virus (qHPV) vaccine against cervical cancer;
o World’s 1st and India’s indigenously developed DNA based Vaccine, ZyCoV-D;
o India’s 1st protein subunit vaccine for COVID-19, CORBEVAXTM;
o India’s indigenously developed mRNA vaccine GEMCOVAC-19™;
o India’s 1st intranasal COVID-19 Vaccine, iNCOVACC;
-
- India’s 1st Omicron booster vaccine based on mRNA Platform, GEMCOVAC- OM.
- First gene therapy clinical trial in India for Hemophilia A: Central Drugs Standard Control Organisation (CDSCO) approved India’s first gene therapy clinical trial for Hemophilia A involving a novel hematopoietic stem cell based lentiviral vector-based gene therapy technology.
- Development of novel blood bag technology: A study group at Institute for Stem Cell Science and Regenerative Medicine (inStem), an autonomous institute of DBT at Bengaluru, developed taurine and acridine containing electrospun-nanofibrous-sheets (Tau-AcrNFS) and showed that these sheets are efficient in decreasing the damage of stored human and mice RBCs ex vivo. Such prophylactic technology may lead to the development of novel blood bags or medical devices, thereby, impacting healthcare by reducing transfusion- related adverse effects.
- National Biopharma Mission (NBM): In alignment with the national missions of ‘'Make-in India’ and “AatmaNirbhar Bharat”, this cabinet approved industry- academia collaborative Mission is focused on enabling and nurturing an ecosystem for affordable biopharmaceutical and biomedical product development. NBM has enabled support for development of 15 vaccine candidates for cholera, influenza, dengue, chikungunya, pneumococcal disease, covid-19 (early development) and related technologies; 21 biosimilar products and related technologies for diabetes, rheumatological and ophthalmic diseases, cancer; 29 medical devices & diagnostics. Notable successes under NBM, during 2023 are:
- Development of a compact, lightweight, next generation MRI Scanner to solve the unmet need of the country. The combination of next generation hardware with software has enabled to successfully introduce a highly disruptive product in the diagnostic imaging space. This is the first Indian company to receive commercial sale and manufacture license from CDSCO, Govt of India.
- Market Authorization Approval (MAA) on 4th September, 2023, for the biosimilar Liraglutide after successful completion of Phase III clinical trial. This will be the First Biosimilar of Victoza in Indian market.
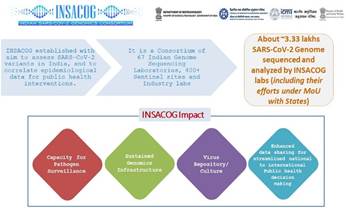
- Indian SARS-CoV-2 Genomic Consortium (INSACOG) consisting of 57 labs and 300+ sentinel sites has sequenced and analysed >3.0 lakh COVID-19 positive samples so far.
- Indian Biological Data Centre (IBDC) is the first National repository for life science data in India, established at Regional centre of Biotechnology (RCB), an autonomous institute of DBT at Faridabad. IBDC is being developed under active collaboration with NIC, NII and ICGEB, New Delhi.
- Data Driven Research to Eradicate TB (Dare2eraD TB) Initiative is the first pan-India initiative at such scale to fully understand the biological characteristics of Mycobacterium tuberculosis (Mtb) and the effect of the mutations on transmission, treatment and disease severity. Nearly 600 samples have been sequenced so far.
Significant Research Insights
- A multicentric study in India was conducted for decoding the genome sequences of bacterial pathogens associated with sepsis, urinary tract infections, and respiratory infections to understand the functional potency associated with Anti-Microbial Resistance (AMR) and its dynamics. Genomic analysis identified several acquired AMR genes (ARGs) that have a pathogen-specific signature. (Proc Natl AcadSci U S A. 2023 Aug 15;120(33):e2305465120. doi: 10.1073/pnas.2305465120.)
- Researchers from THSTI, Faridabad showed that in a K18-hACE2 transgenic (ACE2.Tg) mouse model, IL-9 contributed to and exacerbated viral spread and airway inflammation caused by SARS-CoV-2 infection, and hence represents proof of principle for the development of host-directed therapeutics to mitigate disease severity. (Nat Commun. 2023 Jul 10;14(1):4060. doi: 10.1038/s41467-023-39815-5)
- A novel ¹³C-labelled cyanocobalamin molecule (¹³C-B₁₂) that was biosynthesised allowed for safe testing of vitamin B₁₂ absorption for the first time since the Schilling test (using radioactive-B₁₂) was published 70 years ago. The use of this novel ¹³C-B₁₂ to measure absorption revealed a 'late phase' colonic absorption 10-12 hours after ingestion. This overturns the long-held dogma that B₁₂ absorption only occurs in the small intestine, and can explain why this vitamin deficiency is not profound despite widespread vegetarianism in India. (Bioavailability and daily requirement of vitamin B12 in adult humans: an observational study of its colonic absorption and daily excretion as measured by [13C]- cyanocobalamin kinetics. Am J ClinNutr 2023.
https://www.sciencedirect.com/science/article/pii/S0002916523661180?via%3Dihub)
Inter-Ministerial and International MoUs Signed
- MoU was signed between Armed Forces Medical Services (AFMS) and DBT in presence of Union Minister Dr. Jitendra Singh, on 5th September 2023, to promote healthcare research The MoU will help in pioneering new research in areas like genomics, which have a bearing on lifestyle diseases and emerging diseases like malignant cancer.
- DBT-NSF Research Collaboration Programme: DBT and National Science Foundation (NSF) of the Republic of United States of America signed an Implementation Arrangement (IA) on Research Collaboration on 22nd August, 2023 in New Delhi in the august presence of Dr. Jitendra Singh, Minister of State (Independent Charge), Ministry of Science & Technology.
- DBT and World Intellectual Property Organization (WIPO) signed MoU in presence of Minister Dr Jitendra Singh on 12th October, 2023 for supporting international young professionals to design and create medical devices at DBT Biodesign centers. The initiative is designed to promote Med Tech innovation in youth to respond to global health challenges.
Technologies Developed
- Vacuum impregnation of Iron (Fe) and Zinc (Zn) inside the structure of the lentils was successfully accomplished. This process simultaneously reduces phytate and has the potential to increase in vivo Micronutrient bioavailability.
- A novel wearable electrochemical glove-based analytical device (eGAD) designed especially for detecting the club drug, methamphetamine. The developed sensor shows a 0.1 µg/mL limit of detection and 0.3 µg/mL limit of quantification
- Indian Patent was granted for “Biofilm inhibiting sol-gel composition for coating on substrates and process of preparing”. This biofilm inhibiting sol-gel coating composition imparts antibacterial properties.
- A prototype of a device that can quickly and accurately detect a mixture of bacteria (including S. typhimurium, E. coli, and P. aeruginosa) present in food has been developed. This helps ensure the safety of the food we eat.
- A ceramic membrane integrated anaerobic bioreactor (CMIAR) has been developed for on-site validation of improved technology (iSMAART) and microbial product for effective treatment of textile industry effluent.
- A Coal To Liquid technology (CTL) process for conversion of municipal solid waste into CTL oil has been developed to obtain 80% conversion.
- DBT-BRIC-National Institute of Plant Genomic Research (NIPGR), at New Delhi, through the startup Fruvitec, developed technologies which can enhance shelf life of fruits and vegetables and keep nutritional status intact during storage.
Major highlights of DBT’s Human Resources Development Programme
- Star College Program was implemented in 6 colleges under the Rural category traversing 4 states in the country while 8 colleges have been supported under Urban Category and for the first time a College in Kargil has been selected for financial support.
- 770 students have, been supported in 2023-24 under the Postgraduate Teaching Program (M.Sc./ M. Tech./M.VSc.):.
- 185 fellows were supported in 2023-24 under the DBT-Research Associateship (DBT-RA) Program
- 57 women scientists were supported in 2023 under the Biotechnology Career Advancement and Re-orientation Programme (BioCARe).
North Eastern Region Biotechnology Programme
- Facilities for the generation of certified scion material from Khasi mandarin (Citrus reticulata) and sweet orange have been established at Institute of Horticulture Technology (IHT), Mandira, Assam to develop rootstocks free from Citrus greening bacteria (CGB) and Citrus tristeza virus.
- ‘All-natural’ bamboo and banana leaf-based packaging and carrying devices were developed and studied with Assam lemon, Jambhiri (rough lemon), and Pomelo as model fruits.
- Institute of Bioresources and Sustainable Development (IBSD), Imphal has setup Bioincubator Nurturing Entrepreneurship for Scaling Technologies (BioNEST) incubator to develop women entrepreneurship through orchid floriculture in Meghalaya. Major focus of the project is capacity building and training of women bio-entrepreneurs and farmers from different parts of Ri- Bhoi, Aspirational District of Meghalaya.
Global Bio India 2023 was organized at Bharat Mandapam, Pragati Maidan from 4th-6th December 2023. This largest Biotech Expo of 500+ biotech startups, incubators, industry, and other stakeholders was inaugurated by Dr. Jitendra Singh, Minister of State (I/C) Ministry of Science and Technology. The event witnessed a footfall of 7000+ delegates over 3 days. 29 New Products developed by Biotech Startups were launched in the Global Bio India 2023 event. India BioEconomy Report 2023 launched by Dr Jitendra Singh in the Global Bio-India 2023 showed that India’s bioeconomy has grown to $137 Bn in 2022 with a double digit CAGR consistently for last 6-7 years.
*****
SNC/PK
(Release ID: 1991615)
|