|
Ministry of Science & Technology
Year Ender 2020---Department of Biotechnology (DBT)-M/o S & T
प्रविष्टि तिथि:
19 JAN 2021 12:31PM by PIB Delhi
Salient Achievements 2020-2021
The Department of Biotechnology has made tremendous efforts in promoting Bioscience research, translational education and entrepreneurship. The Department has laid emphasis on the generation of biotech products, processes and technologies for enhanced efficiency, productivity and cost-effectiveness in the areas of agriculture, food and nutritional security; affordable health care and wellness; environmental safety; clean energy and biofuel, bio- manufacturing etc. Skill development programmes have been developed in close coordination with State Governments. The Department has contributed through its various programmes to the National Missions launched by the Prime Minister- Swasth Bharat, Swatch Bharat, Start-up India, Make in India and Digital India. The Department has also risen to the challenge for mitigation of COVID Pandemic by supporting vaccines, therapeutics, diagnostics, genomics, bioreporsitories and a National Platform (NBRIC) for achievement of Atmanirbharta.
Snap shot of achievements: The coordinated efforts of all the stakeholders resulted in supporting more than1642 projects, 2731Scientists and 5145 project fellows, more than 1 Lakh students were trained under Star college scheme, 40 Ramalingaswami Re-entry fellows secured regular appointment, 20,000 rural, SC,ST and Women beneficiaries were supported through Societal programme and more than 25,000 farmers supported thorough Biotech KISAN scheme.
UNATI Atal Jai Anusandhan Mission Programmes
UNATI Atal Jai Anusandhan Mission has been implemented by DBT with a major focus on improved agriculture, affordable healthcare, clean energy and cutting-edge frontier science as per details given below:
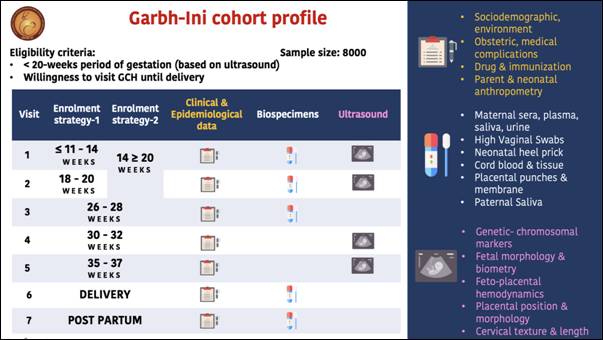
- GARBH-ini (interdisciplinary Group for Advanced Research in BirtH outcomes- DBT India Initiative) study aim to discover molecular risk-markers and generate a risk-prediction algorithm for preterm birth which will facilitate timely referral and care for at-risk mothers, thus saving children’s lives and reducing morbidity. This programme has established a unique pregnancy cohort comprising of more than 8000 women to study Pre Term Birth (PTB). The GARBH-ini platform comprises of a bio-repository (Rakshita) of well characterized clinical phenotypes which has now expanded to include 750,000 bio-specimens and 450,000 ultrasound images.
- The frequency of preterm birth has been found to be around 13%, which is higher than those reported from economically developed (8.6%) or the low-income countries in Northern (7.3%) or Sub-Saharan Africa (12.3%). In addition to the well-known risk factors such as history of preterm birth, short Inter-pregnancy interval and short cervix at 3rd trimester, some less reported factors such as biomass fuel use and exposure to passive smoking as risk factors of PTB have been identified. With respect to BMI at enrolment, both underweight and overweight/obese women were found to have a higher risk of PTB. A first trimester dating formula (called Garbhini-1) has been developed from the biometry of the foetus from mothers enrolled in the cohort which is more sensitive & accurate than global formulae.
- AMR Mission will focus on development of new antibiotics, alternatives to antibiotics and diagnostics. Some of the achievements include:
- National Centre for Microbial Resource (NCMR), National Centre of Cell Sciences, Pune (an Autonomous Institute of DBT), a National Facility to function as a Bio-repository for resistant microbes/infective agents (Bacteria and Fungi)” and to carry out collection, storage, maintenance, preservation and characterization of these microbes across the country.
- A National AMR-specific Pathogen priority list for India to prioritize R&D work in AMR was developed in collaboration with WHO, Country Office, New Delhi
- To address the rising threat of antimicrobial resistance (AMR) with a holistic and multi-sectoral (One Health) approach, “India’s One Health Initiative” to combat problems associated with AMR was launched.
- India has partnered with Global AMR R&D Hub as a member of Board of Members. By partnering, with the Global AMR R&D Hub, the Department will work with all partners to leverage their existing capabilities, resources and collectively focus on new R&D intervention to address drug resistant infections.
Ind-CEPI MISSION is an India centric collaborative mission of DBT aligned to the global initiatives of CEPI (Coalition of Epidemic Preparedness Innovations). DBT is supporting the implementation of the Ind-CEPIs mission “Epidemic preparedness through rapid vaccine development: Support of Indian vaccine development is aligned with the global initiative of the Coalition for Epidemic Preparedness Innovations (CEPI)”, at BIRAC, PSU of DBT.
Ind-CEPI Mission initiated the eCourse Series entitled “Strengthening Clinical Trial Research Capacity in Neighbouring Countries” primarily aimed towards skill development, capacity building and regional networking and coordination. A total of 4-Program 10 sessions series were conducted via online platform with total engagement of more than 750 participants from neighbouring countries like Afghanistan, Bangladesh, Bhutan, Maldives, Mauritius, Nepal and Srilanka.

- UNATI Mission Clean Technologies for Swachh Bharat: DBT has developed various technology platforms designed to convert different solid, liquid and gaseous wastes into renewable fuels, energy and useful products such as food, feed, polymers and chemicals. Under the UNATI Mission, 10 promising clean technologies have been identified for demonstration with DBT support at different sites across India, in collaboration with local stakeholders such as municipalities and other urban local bodies. The identified technologies include bio-methanation, constructed wetland, bio-toilets, chemical & membrane free water purification etc. The first five projects under this initiative were formally launched on 01st Oct 2020, on the eve of Gandhi Jayanti, with an aim to achieve “Swachh Bharat”.
- Fortified Wheat Nutritional Improvement: Anthocyanin rich biofortified coloured wheat lines have been developed by National Agri-Food Biotechnology Institute (NABI), Mohali. NABI has signed 18 MoUs with companies from 9 states and Non-Disclosure agreement with 9 companies from 4 States involved in contract farming and making food products. The program will be addressing micronutrient malnutrition problem and also help the progressive farmers to grow improved wheat lines and encourage entrepreneurship and start-ups.
DBTs response to COVID
Mission COVID Suraksha was announced with a provision of Rs. 900 Cr. to DBT for supporting development of a comprehensive ecosystem for enabling the development of a safe, efficacious and affordable vaccine for COVID-19. The milestone is to accelerate the development of at least 5-6 vaccine candidates and ensure that some of these are brought closer to licensure for consideration of regulatory authorities and for introduction in public health systems.
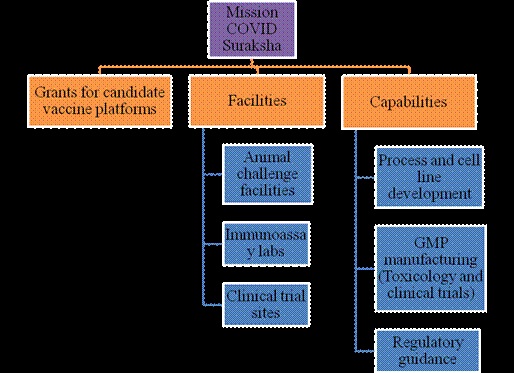
Highlights of major activities include:
- Support for >100 projects in the thematic areas of vaccines, diagnostics and therapeutics
- Enabling 7 vaccine candidates by industry and 8 candidates by academia
- Development of clinical trial sites and centralized laboratories to facilitate vaccine development and leveraging international partnerships.
- COVID 19 testing at 9 DBT AIs, approved as Hubs for their respective City/Regional clusters.
- Rapid scale-up of manufacturing of indigenous COVID-19 diagnostic kits with a production capacity of about15 Lakh kits/day and deployment of nation’s first infectious disease mobile laboratory in Haryana
- 5 COVID19 Biorepositories with more than 40,000 samples available to researchers and industry
- Development of therapeutics from natural products in partnership with M/o AYUSH.
- Nearly 50 BIRAC supported startups have developed innovative products for COVID19.
COVID Vaccines:
- DBT’s National Biopharma Mission supported DNA vaccine, ZyCoV-D, developed by ZydusCadila initiated Phase –III trials
- Some major projects being supported include:
- Phase III trial of rBCG vaccine by Serum Institute of India
- mRNA vaccine (Gennova Biopharmaceuticals Ltd.),
- inactivated rabies virus vector vaccine (Bharat Biotech)
- VesiculoVaxplatform based candidate (AurobindoPharma).
- Partnerships for Accelerating Clinical Trials (PACT): The PACT (Partnerships for Accelerating Clinical Trials) programme has been launched for supporting COVID-19 vaccine development activities in partnering countries. The initiative is being implemented by Biotechnology Industry Research Assistance Council (BIRAC) and Clinical Development Services Agency (CDSA) under the aegis of the National Biopharma Mission and Ind-CEPI Mission of DBT.
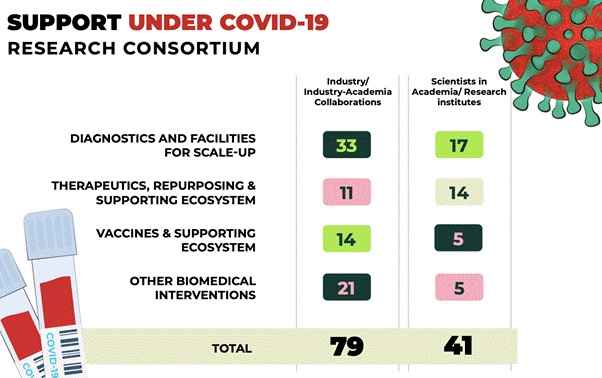
- Supporting COVID-19 research activities through DBT-BIRAC COVID-19 Research Consortium
- The Department of Biotechnology (DBT) and its Public Sector Undertaking, Biotechnology Industry Research Assistance Council (BIRAC), have published a "Request for Proposal (RFP) for COVID-19 Research Consortium" as part of the comprehensive efforts to facilitate development of indigenous research solutions to tackle COVID-19.
- 71 proposals have been recommended in the first phase of the call, focused on the development of different platforms of COVID-19 vaccines and assay systems, diagnostic kits (PCR and antibody-based kits), therapeutic monoclonal antibodies, in vitro models and 3D lung organoid models, hand sanitizers, Personal Protective Equipment (PPE) and low-cost ventilators/respirators, to name a few.
- 114 research proposals were received from the Academic institutions in the area of Diagnostics for financial support against the DBT-BIRAC COVID-19 Research Consortium call. Financial concurrence have been received for all the 17 projects selected for the support. In the thematic area of vaccines, out of 4 projects selected for support, financial concurrence has been received for the 2 projects and 2 projects are under process.
- Continued support will be provided to the recommended projects in the first phase of the call.
- The comprehensive, multi-tiered review and evaluation of thenearly 610 applications received under the follow-up call, came to a completion, with the final Apex Meeting held on July 17, 2020.
- Forty-nine (49) proposals have been recommended funding support under the follow-up call, to fast track R & D efforts for vaccine development, diagnostics, novel therapeutics, repurposing of drugs and any other relevant intervention for control of COVID-19.
- The shortlisted proposals under the follow up call include 7 projects for vaccines, 16 proposals under diagnostics, 10 in the area of therapeutics,2 proposals on drug repurposing, and 14 projects under other interventions. Thirty-one (31) of these 49 proposals have been recommended support under BIRAC and 18 were academia projects recommended under DBT.
- Further, the Department has constituted a COVID-19 Expert Advisory Group to help identify new priority areas and to drive innovative COVID-19 related research efforts.
- The first tranche of release was done to Biological E Ltd to develop a safe, immunogenic & stable vaccine for all populations against the novel coronavirus COVID-19 which is affordable and accessible for all countries. Also the first release was done for Seagull Biosolutions Pvt Ltd for Synthesis, Preclinical characterization & Phase I clinical testing of a novel Active Virosome Vaccine for prevention of COVID-19 infections.
- The first tranche of release was done to Intas Pharmaceutical Ltd for Development of recombinant adeno-associated virus [rAAV] based genetic vaccine for COVID-19.
- The first tranche of release was done to University of Delhi, South Campus for Development of Therapeutic antibodies for COVID-19 in collaboration with Gennova Biopharmaceuticals.
- DBT-BIRAC has prepared 11 clinical trial sites that would help developers test the COVID19 vaccine candidates quickly. Each site will have access to a cohort of about 50000 - 100000 healthy volunteers, who can be tracked for prolonged periods of time.
- Facilitating National efforts for COVID-19 Vaccine development and manufacturing
- DBT is working with the PM-Task force for supporting COVID-19 vaccine development efforts through the PM CARES fund (Prime Minister's Citizen Assistance Relief in Emergency Situations fund.
- DBT-BIRAC supported nation’s first indigenously developed DNA vaccine candidate against COVID-19, ZyCoV-D, by M/s Zydus Cadila has been approved by Drugs Controller General of India (DCGI), for conduct of the Phase III clinical trials.
- DBT-BIRAC held a preliminary discussion with Janssen Pharmaceuticals Ltd., for understanding the strategy for introduction of their COVID-19 vaccine candidate in India.
- Russian Direct Investment Fund (RDIF) announced that Russia will jointly produce Sputnik V vaccine with India. About 300 million doses of the Sputnik V vaccine are proposed to be manufactured in India in 2021.A discussion of Secretary, DBT, and officials of DBT-BIRAC, with Dr. Reddy’s team was held on 4th January, 2021. Dr. Reddy’s team updated on the current status of the clinical trials of Sputnik V in India, the manufacturing capacity, cold-chain logistics from a programmatic stand point.
- The Department proposes to launch the Indian COVID Vaccine Development Mission - Mission COVID Suraksha, with an aim to consolidate the efforts for COVID-19 vaccine development and accelerate development of at least 6 vaccine candidates to ensure their licensure and introduction within the next 12-15 months. The concept note for the mission was also presented in the 10th meeting of the PMO constituted Task Force for Focused Research on Corona Vaccine and other S&T issues held on August 18, 2020.Also, the DPR was submitted to the office of the Hon’ble Minister of S&T. The draft EFC memo for the Indian COVID-19 Vaccine Development Mission “Mission COVID Suraksha” was approved by the Office of the Hon’ble Minister of Science and Technology, Health and Family Welfare and Earth Sciences and by Hon’ble Finance Minister and release of the first tranche of the grant is in process. 2 Requests for Expression of Interest (REoIs) for COVID-19 vaccine development and supporting ecosystem were issued by DBT-BIRAC under “Mission COVID Suraksha – The Indian COVID-19 Vaccine Development Mission”.
- To facilitate Phase III clinical trials of global and national vaccine candidates, five clinical trial sites (INCLEN Trust International, Palwal; KEM, Vadu, Pune; Society for Health Allied Research (SHARE), Hyderabad; National Institute of Epidemiology, Chennai and Christian medical College (CMC), Vellore) and six DHS sites, spread across the Nation are being prepared.
- Meeting between Officials of Qatar and India was held to explore bilateral cooperation for research / training in the areas of diagnostics, therapeutics and vaccines for COVID-19 on 27th October. Member, Health NITI Aayog, Secretary DBT and DG, ICMR participated in the meeting.
- A business meeting of the Joint Working Group (JWG) of the Indo-US Vaccine Action programme (VAP) was held on 15.12.2020, which included a special session on COVID-19 vaccine development efforts in India and USA.
- The Department of Biotechnology had shared a brief update with NITI Aayog, on the efforts for COVID-19 vaccine development and strengthening supporting ecosystem, for inclusion in the statement from India on ACT Accelerator.
- The 12th Meeting of the COVID -19 Working Group of the National Technical Advisory Group on Immunisation (NTAGI) was held on 16thDecember, 2020, whereby there was a discussion on Geographic variation in the outcome of COVID-19 Disease and Contraindications of use of COVID-19 Vaccines.
- In the 4th meeting of Meeting of Standing Working Group on Immunization Vaccine Research Capacity Building held on 16thDecember, 2020, a discussion was held on Research questions on COVID-19 vaccine/s use and implementation.
- Working upon NEGVAC’s interest, DBT/BIRAC officers held a discussion with Austrian Scientists/ Officers on 20th November, 2020, for exploring potential areas of cooperation for COVID-19 research.
- Officials from Vietnam's Office of Science and Technology in India (VOST), Embassy of Vietnam in India, met with DBT, BIRAC officers, on 8th December, 2020, to explore opportunities for cooperation on COVID-19 research activities.
- Information pertaining to the availability of ultra-low temperature storage facilities at DBT Autonomous Institutions of DBT was shared with MoHFW, as part of the exercise to map the availability of cold chain facilities, in the nation. As part of the efforts for mapping the Cold Chain Facilities across the nation, information pertaining to DBT-Autonomous Institutes is being compiled for onward sharing with MoHFW.
- Production capacity of Indian Vaccine manufacturers for their vaccine platforms is being solicited by BIRAC, for consolidating relevant inputs for the COVAX Vaccine Request format shared by MoHFW.
- CDSCO had undertaken an inspection of the laboratory infrastructure and the facilities, at NCCS, Pune and NIAB, Hyderabad, for assessing their functioning as Central Drug Laboratories for COVID-19 vaccine testing. A detailed project report has been requested to be submitted to MoHFW for expansion of the facilities.
MEA accorded 'in principle' approval for financial support for phase 3 trial of COVAXIN in Bangladesh by Bharat Biotech International ltd. (BBIL). Signing of a tripartite HoA with BBIL and ICMR, to finalize the modalities and issues related to the conduct of a COVAXIN based Phase III Efficacy trial in Bangladesh is under consideration. The revised budgetary requirement provided by BBIL has been shared with MEA. The semi-executed tripartite Heads of Agreement was shared with ICMR, to finalize the modalities and issues related to the conduct of a COVAXIN based Phase III Efficacy trial in Bangladesh is under consideration. The proposal for the conduct of clinical trials of indigenously developed inactivated vaccine (COVAXIN) by Bharat Biotech International Ltd.(BBIL), in Bangladesh, is under consideration by the Ministry of External Affairs (MEA). DBT-BIRAC is facilitating the same.
· CDSCO had undertaken an inspection of the laboratory infrastructure and the facilities, at NCCS, Pune and NIAB, Hyderabad, for assessing their functioning as Central Drug Laboratories for COVID-19 vaccine testing. A detailed project report is being prepared for consideration of MoH&FW for expansion of the facilities.
· A discussion of Secretary, DBT with Officials from Myanmar was held on 21st December, 2020 to explore opportunities for association with DBT and procurement and deployment of Indian COVID-19 vaccines in Myanmar.
· A discussion was held with the leadership of CEPI on 21st December 2020 to discuss the potential contribution of CEPI to the clinical development of the RBD based protein subunit vaccine candidate for COVID-19, which was positively reviewed in the third call for proposals given by CEPI for COVID-19 vaccine candidate development.
COVID Therapeutics:
DBT-AYUSH partnership
- Joint network programme, involving DBT AIs and National Medicinal Plants Board (NMPB) to harness the potential of indigenous medicinal plants for development of plant -based therapeutics to treat COVID-19; 50 plants to be screened
DCGI approved First Phytopharma drug
- Phase II clinical trials of AQCH, a phytopharmaceutical drug, developed by DBT-ICGEB along with Sun Pharma initiated.
Immunoglobulin based therapeutics
- Immunotherapy of COVID infected patients using therapeutic antibodies from Human or Equine sources
Clinical trial for equine immunoglobulin therapy to start shortly
- Resources for drug screening
- Organoid technology in vitro platform for drug screening and identification of new drug targets
DBT-RCB has set an in vitro cell culture-based assay to test the antiviral activity of potential molecules against SARS-CoV2. Services have been widely utilized by the academia and industry. Cytotoxicity Testing and anti-viral testing was done on 679 and 313 samples respectively
COVID Diagnostics:
- Hon’ble Minister for for Science & Technology, Health & Family Welfare and Earth Sciences, announced the successful manufacturing of 100 lakh diagnostic kits for COVID-19, by DBT-Andhra Med Tech Zone (AMTZ) National COMManD Consortium [COVID MedTech.
- Hon’ble Minister for for Science & Technology, Health & Family Welfare and Earth Sciences launched the country’s first mobile I-Lab (Infectious disease diagnostic lab) for last mile coronavirus (COVID-19) testing access on 18th June 2020. The lab will be deployed in interior, inaccessible parts of the country and has the capability to perform 25 RT-PCR tests a day, 300 ELISA tests a day and additional tests for TB, HIV as per CGHS (Central Government Health Scheme) rates.
- First Indigenous kit for diagnosis of COVID-19 developed by a BIRAC supported Startup MyLab, Pune - producing nearly one lakh kits per week.
- Andhra Pradesh MedTech Zone (AMTZ): Shared facility to manufacture diagnostic kits, ventilators and imaging equipment. Manufacturing capacity of 3 lakh RT-PCR kits/month, 1 lakh RNA extraction kits/ month and 1 lakh Viral Transport Medium (VTM)/month.
- Dhiti Life Sciences - Fully indigenous Antibody detection kit commercialized.
- Ubio – Antibody detection kit developed and is commercialised.
- Molecular Transport Medium (MTM) and Nucleic acid extraction kits developed by Huwel Lifesciencs available in market.
- DBT-NIAB developed Electrochemical device for ultrasensitive and rapid diagnosis of SARS-CoV-2. MoU was signed for the transfer of technology to M/s Biogenex Life Sciences Private Limited.
- DBT-THSTI developed the first Aptamer based SARS-COV2 detection assay. The Technology was transferred to Molbio Diagnostics Pvt Ltd.
- COVID testing services: 23.83 lakh samples tested as on 1st January, 2021, across the hubs.
Covid Genomics:
- DBT-AI consortium successfully completed PAN-India 1000 Genome sequencing of SARS- CoV-2 here in a record time of 3 months. Initial results indicated that , there is a predominance of the A2a haplotype (20A/B/C) with D614G mutation, which is globally reported to be associated with enhanced transmission efficiency.
- Indian SARS-CoV-2 Genomic Consortium (INSACOG) has been launched, to ascertain the status of new variant of SARS-CoV-2 in the country. The Consortium is being coordinated by DBT along with MoH&FW, ICMR, Delhi and CSIR. The consortium aims to establish sentinel surveillance for early detection of genomic variants with public health implication.
Augmentation of self reliance and support to Start-ups:
- NBRIC is a nation-wide effort for convergence of indigenous resources, products and services towards developing diagnostics, vaccines and therapeutics for COVID-19 and beyond for self-reliance in India’s biomedical capabilities.

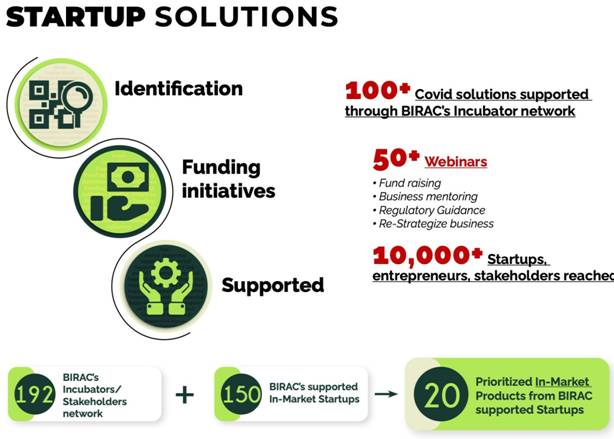
COVID Start-up solutions:
- 100+ Covid solutions supported through BIRAC’s Incubator network
- 10000+ Start ups, entrepreneurs and stakeholders were reached through online webinars and wide consultations
COVID Biorepositories:
- Four Biorepositories have been established at DBT AIs, viz.,Translational Health Science and Technology Institute (THSTI), Faridabad (for clinical samples), Regional Centre for Biotechnology (RCB), Faridabad (viral repository), Institute of Life Sciences (ILS), Bhubaneshwar and Institute for Stem Cell Science and Regenerative Medicine (inStem), Bengaluru, (for oro/nasopharyngeal swabs, sputum, blood, urine and stool samples).Sharing of the archived biospecimens is hoped to enable accelerated COVID-19 related research and innovation.
-
Sectoral Achievements:
Health:
- National Biopharma Mission: The first ever Industry-Academia mission “Industry-Academia Collaborative National Biopharma mission: the Mission for Accelerating Discovery Research to Early Development for Biopharmaceuticals – “Innovate in India (i3) Empowering biotech entrepreneurs & accelerating inclusive innovation “ to accelerate biopharmaceutical development in India. Major achievements of the mission are highlighted below:
|
Product Development
|
Shared Facilities
|
Skill Development
|
Technology Transfer
|
|
Vaccines
11 vaccine candidates for Flu, Cholera, Dengue, Pneumonia, and COVID, under different stages of development are being supported
|
National Facilities
Creation of GLP, GMP, GCLP facilities besides cell line repositories and facilities for medical device testing and prototyping. Establishment of 19 national facilities are ongoing, out of which 04 are already operational.
|
Trainings & Workshops
1406 candidates have been trained in the areas of clinical research, regulatory compliances, technology transfer, biopharmaceuticals and medical devices. 37.12% of the participants were female.
|
Tech Transfer Offices (TTO)
Five TTO’s have been established with a view to strengthen the technology transfer capacity of the country.
|
|
Biotherapeutic mAbs
About 12 mAbs which are not presently existing in Indian market and 03 clones for diseases like cancer, diabetes, psoriatic arthritis, wet macular degeneration and lung infections caused by RSV are being supported. Additionally, one antibody-drug conjugate (ADC), one novel biologic, one recombinant monoclonal and one bio-better are also being supported.
|
Translational Research Consortia (TRC)
The Mission has supported two consortia
(i.) TRC for Dengue: 3 clinical sites and 5 premier institutes across the country led by ICGEB, Delhi.
(ii.) TRC for Chikungunya (CHKV): 4 hospitals and 3 premier research institutes across the county, led by Manipal Academy of Higher Education.
|
|
|
|
Device & Diagnostics
Currently 17 devices and 13 diagnostics are being supported. Support is being given for development of products in the areas of hospital-use equipment, diagnostic imaging, implants, wound-care products etc. Many diagnostic devices and reagents used for diagnostic kits are also supported including Molecular diagnostics, ELISA, LFT, sample transport reagents etc.
|
Clinical Trial Networks (CTN)
CTN for hospital-based trials in patients for testing biologicals in different specialties of oncology, diabetology, rheumatology and ophthalmology. Currently, 05 consortia including 36 hospitals are being supported. Strengthening the capacity of vaccine clinical trials, in already existing Demographic Surveillance sites and establishing new sites is another major focus area. Ten (10) Field Sites have been prepared for the same.
|
|
|
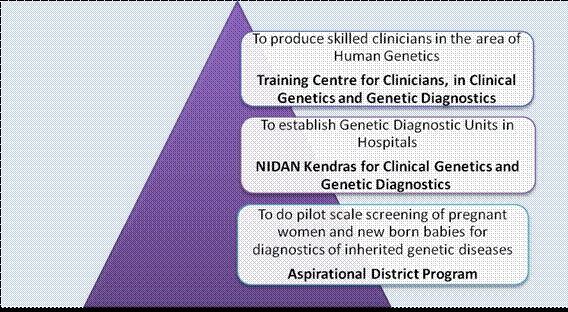
- Unique Methods of Management of Inherited Disorders (UMMID) Initiative: The Department launched ‘UMMID’ initiative to tackle inherited genetic diseases in newborns. Under the pilot phase of the UMMID initiative, the Department has supported the establishment of 5 genetic laboratories, 7 training centres for training clinicians in diagnosis and management of genetic diseases and screening of pregnant women and neonates in seven aspirational districts. Seven Aspirational Districts namely Mewat, Haryana; Yadgir, Karnataka; Haridwar, Uttarakhand; Washim & Nandurbar, Maharashtra; Ranchi, Jharkhand; Shrawasti, Uttar Pradesh have been identified for screening of pregnant women and newborn babies for diagnostics of inherited genetic diseases to provide comprehensive clinical care. So far about 34000 pregnant women and 16000 new born babies have been screened

-
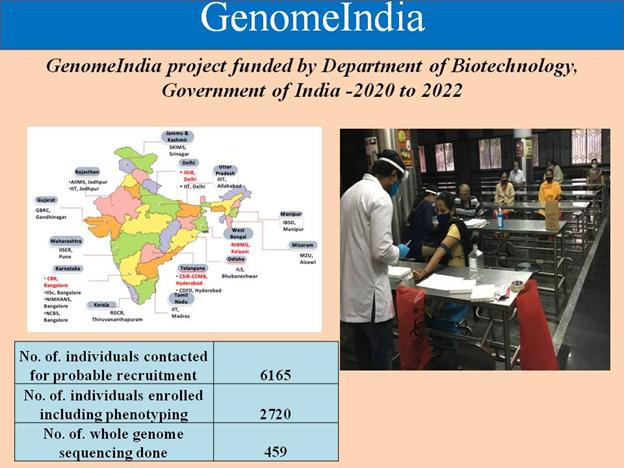
- Pan India Genome India was initiated a pan India 20 institutions consortium for cataloguing the genetic variation in Indian population with a goal for whole genome sequencing and subsequent data analysis of 10,000 individuals representing the country’s diverse population to help build an exhaustive catalogue of genetic variations in Indian population, and to aid in the design of a genome wide association chip for Indian population. As on 8th Jan 2021, 6165 individuals were contacted for probable recruitment in study, 2720 individuals have been enrolled for recruitment as study participants into the project. 549 samples have undergone WGS and genome wide high-throughput genotyping. Analysis of genetic variants calling in these genomes is in progress.
- PLUTO: A modular, portable, and compact robot for training different hand functions (for hand neurorehabilitation) was developed.
- The technology for Sonography Training Simulator has been commercialized with Merkel Haptic Systems. The Software converts CT/MRI data to3D modals.

- A Portable Raman Spectrometer with high efficiency SERS multifiber probes was developed with classification accuracy of 93% in differentiating malignant, pre-malignant and healthy tissue samples.
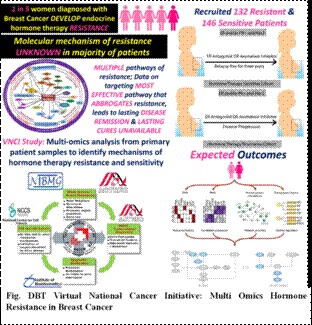
- DBT VNCI- Hormone resistant Breast Cancer study with TMH Mumbai, ACTREC Mumbai, NIBMG, Kalyani and NCCS, Pune has recruited 131 patients in therapy sensitive and 142 patients in therapy resistant cohort. Preliminary analysis has identified known (genetic aberrations in Estrogen gene ESR1) as well as potentially novel genetic aberrations that play a role in resistance to hormone therapy.
Agriculture and Allied Areas:
Agriculture Biotechnology
- Mission mode programme on “Minor Oilseeds of India Origin” in various crops was launched which is aimed at sequencing/re-sequencing and phenotypic characterization of available germplasm resources of Minor Oilseeds (Sesame, Linseed, Safflower and Niger) in the country along with exotic lines from diverse agro climatic regions & elite lines of International Institutes.
- Mission mode programme on “Characterization of Genetic Resources” in various crops was initiated for sequencing/re-sequencing and phenotypic characterization of available germplasm resources of Chickpea in country along with exotic lines from diverse agro climatic regions & elite lines of International Institutes has been supported.
- “India-UK crop science fellowship (INcrops)” was launched to create a skill set of trained manpower in cutting edge technologies in crop sciences. It is aimed to send ten (10) post-doctoral fellows per year to the UK institutions.
- DBT-NGGF “National Genotyping and Genomics Facility” (NGGF) a “single-window service system” for advanced genomics technology services was established to offer affordable and competitive genotyping and genomics services to Public and Private Institutions. Crop breeding companies and seed companies (205) involved in regular breeding process and activities will be a source of revenue for this facility.

A few crop wise major achievements are placed below:
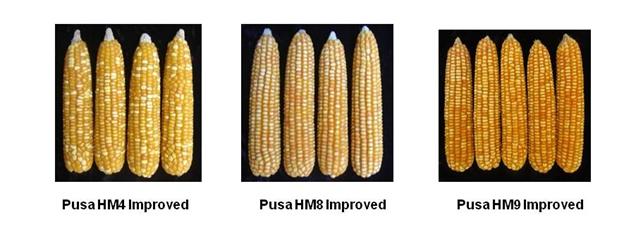
- Three improved maize hybrids have been developed by Enrichment of Nutritional Quality in Maize through Molecular Breeding.
- Pusa HM4 Improved in terms of protein quality and possesses high tryptophan (0.91%) and lysine (3.62%). It has been released and notified for North Western Plain Zone.
- Pusa HM8 Improved in terms of protein quality and possesses high tryptophan (1.06%) and lysine (4.18%). It has been released and notified for Peninsular Zone [Maharashtra, Karnataka, Andhra Pradesh, Telangana & Tamil Nadu].
- Pusa HM9 Improved in terms of protein quality and possesses high tryptophan (0.68%) and lysine (2.97%). It has been released and notified for North Eastern Plain Zone [Bihar, Jharkhand, Odisha, West Bengal & Eastern Uttar Pradesh].
Animal Biotechnology:
- Urine based early pregnancy diagnostic kit for cattle and buffalo.
- 6 surgical grade stainless steel TPLO plates for cranial cruciate ligament rupture surgery
- PGC based method was developed for construction of chimeric chicken.
- Nano-Immuno Rapid Test to detect Mycobacterium avium subspecies paratuberculosis in milk samples
- New method of diagnosis of Johne’s disease in the domestic live stocks
|
|
Process/Product/ Technology Developed:
| |
| |
Process/Product/Technology Transferred to User Agency/ Industry/ Stakeholders:
- Transfer of technology of Brucella ∆S19 mutant per vaccine to industry.
Process/Product/Technology Commercialized:
-
- Low Passage High Titre Canine Parvo virus (CPV-2b) Vaccine (TRPVB)
- Viroclean (Germicidal hand rub) (TRPVB
|
|
- Consortium for One Health to address Zoonotic and Transboundary Diseases in India was established to contribute to (a) the availability of state-wise and nation-wide data on the selected zoonotic diseases and transboundary animal diseases (TADs), (b) the validation of indigenous tests for use in the field, (c) collection of valuable reference (positive and negative) sera banks, (d) the development of platforms such as monoclonal antibody facility and generation of laboratory animal models, (e) the establishment of a network of veterinary and medical centres and laboratories to work together, (f) initiate investment towards border security in terms of incursion of pathogens, and (g) a roadmap to provide strategy for a continuing endeavour and a sustainability pathway for One Health programme in India.
- Genomics for conservation of indigenous cattle breeds and for enhancing milk yield: The whole genome sequencing data (coverage ~ 30X) of 176 animals was generated including 20 animals from each of five major breeds - Gir, Tharparkar, Kankrej, Red Sindhi and Sahiwal. The draft genome sequence for 4 major milch breeds (Gir, Sahiwal, Tharparkar & Kankrej) has been assembled.
Aquaculture:
- “Germplasm resource centre for marine ornamental invertebrates” in CMLRE field station at Agatti Island, Lakshadweep. So far, more than 280 individuals of ornamental shrimps were collected and stocked in the centre. Among the shrimps, Thitarodeshaina nensis was reported for the first time from Indian waters. In addition, 70 tentacle sea anemones were also collected and work on their asexual reproduction is being carried out. Brood stock development was successfully achieved for 5 species of shrimps.
- Reference proteome map developed for Rohu by proteomic profiling resulted in the identification of more than 10,000 unique proteins. The resulting reference proteome map can be used as a baseline for further omics research in rohu.
- A whole virus vaccine against nodavirus of fish was produced using seabass kidney and grouper eye cell lines.
Energy, Bioresources, Environment and Forest Biotechnology:
- Launch of Clean Tech Demo Park: DBT-BIRAC Clean Tech Demo Park at Barapullah drain site, near Sundial Park, Sarai Kale Khan, was inaugurated by Hon’ble Minister for Science & Technology, Health & Family Welfare, and Earth Sciences on 08 Oct 2020. The DBT-BIRAC Clean Tech Demo Park will be used to demonstrate innovative Waste-to-Value technologies with support from DBT and BIRAC.
- 2G Ethanol Technology has been demonstrated at 1 TPD scale, with Steam explosion pilot plant at 300 Kg/Day scale.
- The DBT-IOC Centre has developed a fungal mutant that produced about 12 FPU/ml of culture broth at 5 Litre scale. This enzyme technology has been scaled up to 5,000 L bioreactor with more than 10 FPU/ml enzyme titer value.
- Hyperthermophile enzyme hydrolase research centre (HERC) has been set up at IISER Mohali as a repository for thermophilic and hyperthermophilic organisms and their enzymes. This centre has cloned and produced several carbohydrate active enzymes which will be evaluated for use in 2G Ethanol and biorefinery applications.
- Waste to Energy: Two demonstration plants successfully commissioned and operated for converting municipal solid waste (bioorganic fraction) into energy by improved biomethanation process at the scale of 3 Tons-7 Tons/day. The trial is ongoing for operation at full capacity of plants located at Hyderabad and Goa.
- Engagement with MI Community:
- India has been actively participating in MI Ministerial(s) and at the 5th Mission Innovation Ministerial (MI-5) held virtually on 23rd September 2020 hosted by Kingdom of Saudi Arabia was attended by a high-level delegation led by the Hon’ble Minister of Science & Technology, Earth Sciences and Health and Family Welfare Dr Harsh Vardhan, Secretary DBT Dr Renu Swarup and senior officials from DBT and DST.
- The meeting reaffirmed India’s commitment via Joint Member Statement to accelerate the pace of innovation and facilitate clean energy transitions by advancing the solutions and technologies needed to support national goals. Details can be accessed at http://mission-innovation-india.net/mi-5/
- Customized constructed wetland (CW) technologies have been developed for treatment of household sewage and industrial effluents including distillery waste, paper and pulp mill waste and textile industry waste.
- A system for removal of floating debris from the Barapullah drain has been installed in collaboration with DESMI, Denmark, resulting in removal of 175 tons of waste and preventing it from flowing into Yamuna River.
- Under the LOTUSHR project in collaboration with Netherlands, a toolbox of wastewater treatment technologies has been established on site at Barapullah drain to facilitate selection of treatment combinations for optimum treatment of wastewater.
- Phytopharmaceutical Mission for North East Region of India was launched by DBT to promote development of phytopharmaceuticals in North East Region. A QC and QA facility for medicinal plants has been already established and cGMP facility for pilot-scale extraction for phytopharmaceutical products from the medicinal Plants of NE India is being set up. In collaboration with CSIR and ICMR, a pipeline of phytopharmaceutical drugs has been developed.
- Turmeric Mission programme was launched to generate high-quality raw material for developing nutraceutical products / dietary supplements from turmeric for global market as well as developing curcuminoids / curcumin-based therapeutics for various disease segments.
- DBT has signed a Memorandum of Understanding (MoU) with National Medicinal Plant Board, Ministry of AYUSH for Inter-Ministerial Cooperation on biotechnological intervention in AYUSH sector and to have a platform for exchange of information between the NMPB, Ministry of AYUSH and DBT. Proposals are being developed for joint funding and implementation by Ministry of AYUSH and DBT.
- Phytopharmaceutical product for Bovine Mastitis was developed jointly by CSIR-IIIM, Jammu; ICAR-CIRG, Mathura; and ICAR-NDRI, Karnal. Six best extract were selected from four medicinal plants (Terminalia bellerica, Piper betle, Boswellia serrata and Bergenia ciliata) for the development of three topical gel products as anti-infective and anti-inflammatory for bovine Mastitis.
- National Centre for Microbial Resource (NCMR): A total of 4,778 cultures have been identified. The total number of cultures deposited under the ‘General deposit’ category has reached 5,942, of which 3,500 are listed in the online catalogue after authentication, and are accessible to academia and industry. A total of 21 peer-reviewed research articles have been published during this year. Three new species of bacteria were described, namely, Rhizobium indicum sp. nov., isolated from the pea root nodules collected from Indian trans-Himalayas, Klebsiella indica sp. nov., isolated from a tomato skin and Pseudomonas lalkuanensis sp.
- Indian Bioresource Information Network (IBIN) created to bring together all the available database and information on the bio-resources and biodiversity of the country in one platform. The satellite telemetry tracking of Jacobin Cuckoo is a part of IBIN project in collaboration with Wildlife Institute of India to monitor the changes in Indian monsoon patterns and could help in planning the conservation and climate monitoring programmes.
Knowledge Generation:
- Indian Biological Data Centre (IBDC) was established by DBT for deposition, storage, annotation and sharing of biological data generated in the country through extensive funding from various Government Organizations. The IBDC will enable life science researchers to deposit biological data in a central repository and thus safeguard data generated using public resources from loss. It will perform quality control, curation, and annotation of data. These efforts will help establish benchmarks for the quality of data deposited and thus improve the quality of experimental research conducted in the country.

- Artificial Intelligence (AI) tools for affordable and accessible Healthcare - Big Data and Genomics in areas of cancer, tuberculosis and pulmonary diseases, diabetic & cardiovascular diseases, ophthalmological diseases, neurological disorders and methods/ drug development were supported. An Imaging BioBank for Cancer was also established to develop AI tools and database for advance research in cancer and will also be aimed at cancer diagnosis/ prognosis and cancer care.
- Revamping of Biotechnology Information System (BTIS) Network: New set of centres of BTISNet are being funded during this financial year-2020-21 in the areas of Structural Bioinformatics/ Drug discovery/ Drug development/ Cheminformatics, Machine Learning, Genome Informatics/ Metagenomics/ Systems Bio/Microbial, Agriculture/ Plants/ Animal, Human diseases/ Disease informatics, Biodiversity, Proteomics and Metabolomics. These centers (Spokes) will be linked to IBDC (Hub).
- GenoVault: “A Cloud based Genomics Repository”: GenoVault (Cloud based Genomics Repository) was launched in the Accelerating Biology 2020: SNiPs to SPiNs, held in Pune, in the presence of Dr. Renu Swarup, Secretary DBT, Dr Hemant Darabari, Director-General CDAC, Padma Bhushan Dr Vijay Bhatkar, Chancellor, Nalanda University, Prof. Laxmikant Kale, University of Illinois, USA and other international and national dignitaries. This cloud-based genomic repository enables quick archival and retrieval tools along with extremely powerful analytical engines. GenoVault is a first India’s Genomics Repository based on OpenStack cloud. This centralized repository would be of enormous importance for healthcare and would of great use in personalized medicine.
- Bio-liposome mediated CRISPR-Cas9 delivery system: A novel bio-inspired lipid nanocarrier system for efficient intracellular delivery of CRISPR-Cas9-based genome editing tools has been developed. The development of bio-inspired lipid nanocarrier delivery system would open new vistas for devising novel gene therapy-based therapeutic solutions for various rare and genetic diseases.
- Release of “Guidelines for Evaluation of Nano based Agri-input &Food Products in India”: Dr. Harsh Vardhan, Hon’ble, Union Minister of health and Family Welfare, Earth Sciences and Science and Technology along with Shri Narendra Singh Tomar, Hon’ble Union Minister of Agriculture & Farmers Welfare and Hon’ble Union Minister of State Shri Parshottam Khodabhai Rupala. Hon’ble, Union Minster of Agriculture and Farmers Welfare have released the “Guidelines for evaluation of nano-based agri-input and food products in India” on 7th July 2020. These ‘Guidelines’ are applicable to nano-agri-input products (NAIPs) and nano-agri products (NAPs). These ‘Guidelines’ also apply to nano composites and sensors made from NMs and those that require direct contact with crops, food and feed for data acquisitions.
DBT-HRD Salient Achievements 2020-21:
- Number of courses supported under DBT PG Teaching Programme during 15th Finance Commission (2020-21 to 2024-25): 70
- Number of Ongoing DBT JRF Fellows: 767
- Number of new DBT JRF Fellows selected in 2020-21: 477 (Category-I)
- Number of Ongoing DBT RA fellows: 183
- Number of new DBT-RA fellows selected in 2020-21: 36
- DBT JRF and Graduate Aptitude Test in Biotechnology (GAT-B) conducted by DBT at 59 centers all across the Country.
DBT-Skill Vigyan Programme:
- Implemented in six states. Proposals of three more states approved for implementation. Proposals of 25 State/UTs under evaluation.
- Support continued to 15 Skill Development PG Diploma/certificate courses
DBT Star College Scheme
- 240 undergraduate colleges across the country are currently being supported under the DBT Star College Scheme. During this year 52 new colleges were supported, of which 33 were urban colleges and remaining 19 were located in rural areas. A total of 7 colleges were accorded with the Star Status based on their performance; 6 urban and 1 rural college.
- Department of Biotechnology is promoting Women in Science through BioCARe (Biotechnology Career Advancement and Re-orientation) Programme to enhance their enriched and valuable contributions and supports to enhance their employability. This year, 3 women scientist got permanent employment after availing the BioCARe grant.
Infrastructure
- 53,685 users have utilized the SAHAJ portal (until September 2020) generating revenue around Rs. 2.00 crores despite the restrictions of 4 months due to Covid-19 pandemic. Further analysis revealed that while 38,501 users were from the institutes where this equipment are hosted, 5284 users were external users from other institutes and industry partners.
· Initiation of 3rd Phase of the “Access to Structural Biology Facilities at ESRF, France”: The European Synchrotron Radiation Facility (ESRF), located in Grenoble, France, is the world's most intense X-ray source and a centre of excellence for fundamental and innovation-driven research in condensed and living matter science. More than 300 Ph.D students and 1.52 lakhs researchers have utilized the services till date and has led to publication of more than 500 high impact research articles.
Conference, Travel, Exhibition and Popular Lectures (CTEP)
- 122 proposals were received for support under Conference, Travel, Exhibition and Popular Lectures prior to the pandemic, 101 proposals were recommended for support and Rs. 43.62 lakhs were released for funding.
Societal Programme
- 5 new Rural Bioresource Complexes, 1 each in the states of Karnataka, Maharashtra, Tamil Nadu and 2 in the state of Andhra Pradesh were established. These centers will benefit around 4000 individuals from the SC/ST community, rural unemployed youth and women folk from the Aspirational districts of Yadgir, Akola & Washim, Virudhnagar , Vizianagram and Kadapa.
- Bio fortified antioxidant rich colored wheat cultivation under organic and agronomic supplementation (Fe, Zn, Protein) implemented in the Aspirational district of Moga and Ferozepur, Punjab has helped farmers to garner Rs. 2000 to Rs. 4000 higher income per acre after the sale of their produce.
- DBT has funded 53 projects for benefitting around 20,000 individuals from rural areas, Aspirational districts, womenfolk, youth, SC/ST and socially backward communities. Out of these beneficiaries, 8518 individuals have been supported under projects implemented under the Aspirational districts program.
Biotech-KISAN has created a platform in each of 15 agro-climatic zones of the country, which aims to connect farmers and scientists to promote Agriculture Innovation and take the new interventions to the farmers and farms. Biotech-KISAN Hubs have been established at pilot-scale level in all agro-climatic zones. The programme has now been scaled up and expanded its activities covering 105 Aspirational Districts in the country. The programme will lead to link the farmers with the latest scientific innovations with a view to enhance agricultural production and increasing farmers’ income. The programme has introduced 200 interventions for the benefit of farming community and more than 25000 farmers have been benefitted so far.
International Cooperation:
- The department also strengthened its partnership with the Academy of Medical Sciences (AMS), UK to launch the AMS-DBT Newton International Fellowship (NIF). This unique opportunity will offer a 3 year fully supported post-doctoral fellowship for Indian scholars to pursue clinical and patient-oriented research in the UK and India under joint mentorship. This fellowship will facilitate the transition of exceptional scholars from budding young researchers to early-career independent researchers with strong international mentorship and training experience.
- Focusing on the threat of climate change, the department collaborated with European Union for the “Green Deal” under Horizon 2020 framework to help build a low-carbon, climate-resilient future. The department will support joint research and innovation initiatives focused on Clean, affordable and secure energy, Industry for a clean and circular economy, Farm-to-Fork Strategy, Biodiversity and ecosystem services, and Zero-pollution, toxic-free environment.
- Multilateral collaboration:
- Eureka-Global Stars India initiative is mandated to cooperate in such a manner that Indian academic/research institution can collaborate with industrial partner from Eureka member country leading to translation based research. This collaboration led to launch of two joint calls with Eureka member countries in the area of one health in 2018 and Key Enabling Technologies for Healthcare, Agriculture and Water in 2020 respectively.
- To match the pace of Indian scientific communities participation at the international level in the attainment of targets of United Nation’s Sustainable Development Goals, a Memorandum of Understanding (MoU) was signed between the Department of Business Innovation and Skills, Government of the United Kingdom of Great Britain and Northern Ireland and Ministry of Science & Technology, Government of India for Newton-Bhabha Programme (A UK-India Research and Innovation Partnership) on 12th November, 2014. This led to the announcement of a joint call for proposal under the TaSE (towards a sustainable Earth) initiative for “Human-Environment Interactions and the Sustainable Development Goals”.
- Department of Biotechnology (DBT) with the Department of Science and Technology (DST) as a coordinating agency under a joint multilateral collaborative initiative announced a joint call on “Response to COVID-19 pandemic” under BRICS-2020 STI Framework Programme.
NER Program
- With the aim to promote sustainable development and utilization of the unique bioresources of NER and linking it with socio-economic upliftment of the region, the Department announced programme on “Development and Utilization of Bioresources of North East Region for Generating Livelihood Security and Entrepreneurship”. The programme aims to demonstrate and deploy the entrepreneurial outcomes of the already available technologies with proven effectiveness, developed on the priority resources of NER.
- The Department has announced a programme on “Understanding Chemical Ecology of the North East Region. The programme aims to bring together researchers in complementary disciplines to explore the role chemical signals plays in shaping the unique ecosystems of the NER by studying interactions at the molecular level using advanced metablomics tools to answer age old questions by studying how organisms communicate with each other via. chemical signals and discovering novel molecules for improving human life and environment.
|
Personals Trained in NER
- 678 students trained as JRF/SRF, 68 Post Doctoral fellows
- 33 PhD students were awarded PhD degrees under Chemical Ecology Programme and CoE-NECAB at AAU, Jorhat
- 35 NER researchers trained at NECAB at IIT-Guwahati in Healthcare Engineering
|
|
Publications
- 46 publications in peer-reviewed journals
|
|
Patents filed/Granted
- Eight patent applications filed before the Indian Patent Office
- Two patents granted (one International and one national)
|
|
Technologies transferred
Bioformulations developed at CoE AAU Jorhat –NECAB include Talc based formulations (Bio-Time; Biozin-PTB; Bio-Sona; Biollium, Bio-Meta; Bio-Zium; Bio-veer; Biogreen; Biogreen-L) and Organic based (Biofor-Pf). MoU was signed for transferring Bio-input (Biopesticide production technology) Technology to the three companies: VRS Agritech, Guwahati, School of Livelihood & Rural Development (SLRD), Shillong, M/s Orgaman R&D Division, Jorhat.
|
|
Entrepreneurship Development
- Technology Incubation Centre for Entrepreneurship Development on Mushroom Culture & Farming developed at Bodoland University, Kokrajhar, Assam: A total of 2700 farmers and 48 small scale entrepreneurs have been trained in mushroom cultivation in Bodoland District of Assam
- 150 farmers trained in cultivation of Strawberry with improved Agro-technology in Farmers field in Meghalaya
- 1000 farmers trained in Dendrobium cut flower production systems in Assam and Meghalaya
|
Biotechnology Industry Research Assistant Council (BIRAC)
- Biotechnology Industry Research Assistant Council (BIRAC)BIRAC has supported technology -driven 1500+ Entrepreneurs, Startups and SMEs; through various operational models of cooperation infusing INR 1144.77 Cr funds and mobilized INR 981.05 Cr funds through partnering with Public, Corporates, Philanthropic organizations at the national and international level to harness growth synergies for the of Ecosystem. A network of 50 Bio-incubators has been built creating an Incubation space of 5,48,000+ sq. ft. with high-end infrastructure providing access to instrumentation, technical, IP, legal and business mentorship for biotech startups. This has helped innovators to create a pool of intellectual wealth (225+ IP filed) and has supported in the launch of 150 product and technologies in the market.
-
- BIRAC’s Incubation Centre BioNEST Network provides the critical nurturing ground for the Biotech Startups across the country as depicted in the cluster map. The number of startups are expected to grow to 10,000 by 2024.
-
- The BioNEST incubators of BIRAC have led to the development of 130+ COVID-19 related solutions have been developed by the start-ups supported. More than 300+ virtual events have been conducted since March 2020 till date by BioNEST incubators and 25,000+ participants have been benefited from the same.

- A Fast-Track internal review committee was constituted in April 2020 at BIRAC to provide fast track support to health-tech start-up solutions ready for immediate deployment (0-3 months), that can be supported under COVID fund. The health-tech start-ups supported under fast-track review are: Aarna Biomedical Products (Suraksha Full Body Coverage Kit), Alpha Corpuscles (Face shields), MicroGO (GO Assure- automated hand hygiene device), Ubiqare Health (Specialty mobility healthcare platform), Ayu Devices (Bluetooth Enabled Digital Stethoscope for COVID-19), Health Sensei (Reduced Contact Central or Remote Monitoring solution) and DNA Xperts (COVID 19 Diagnostic solutions). Additionally, BIRAC has also supported two Co-funding partners (IKP and C-CAMP) for in turn funding up to 25 Start-ups under the BIRAC’s mandate to foster market deployment of innovative solutions addressing COVID-19 challenges.
- BIRAC launched i4 (Intensifying the Impact of Industrial Innovation) to support biotechnological product/technology development by strengthening R&D capabilities of start-ups/companies/LLPs. The programme provides impetus for pulling the translational ideas past PoC and taking them further along the innovation chain for validation, scale-up, demonstration and pre-commercialization of products and technologies.
- Grand Challenges India: has launched 20 programs across diverse themes in public health domains, since its inception. The programs have received 3000+ applications and more than 120 projects have been recommended for funding support. All the supported programs/projects are aimed at identifying health care innovations that will enable the goal of equitable health care in India and beyond.
- Grand Challenges Annual Meeting 2020: The Grand Challenges Annual Meeting 2020 was held from 19th -21st October, 2020 and brought together scientific leaders, researchers and policymakers from around the world to impact and create transformational solutions to address global health and development problems. Hon’ble Prime Minister inaugurated the Grand Challenges Annual Meeting 2020 and delivered the keynote address. He praised the Grand Challenges program and stressed on the efforts taken against the COVID-19 pandemic through global collaborations and focused on India’s vaccine manufacturing capabilities. He also stressed that platforms such as Grand Challenges are crucial to creating an enabling mechanism which brings researchers together and will provide better opportunity for greater collaborations and solutions.
`
DBT - Autonomous Institutions
16 Autonomous Institutions are functioning under the aegis of the Department of Biotechnology. These institutions are pursuing basic, discovery and translational research in line with the National missions in the areas of Agriculture Biotechnology, Animal Biotechnology and health, Medical Biotechnology, Clean energy and Bioresourse development, Secondary Agriculture etc. The institutions also have a mandate of human resource development and societal outreach. Some of the achievements during the year are given below:
DBT-National Institute of Immunology (DBT-NII) has developed Mycobacterium indicuspranii based leprosy vaccine and immunomodulator. This is being marketed by Cadila Pharmaceuticals, Ahmedabad as Immuvac. DBT-NII has published 85 research papers in reputed journals, was granted 5 patents and filed 11 patent applications. In order to protect intellectual property rights of the research endeavors, two trademarks, ASPAGNII and PANII have been filed for vaccine and biotechnological composition respectively. During this year, the Institute has also sealed a Technology Transfer Agreement with Vitane Biologicals.
DBT-Center of Innovative and Applied Bioprocessing (DBT-CIAB) has received the granted status of its 7 patents on various bioprocess based technologies/products developed by the institute. 5 new technologies were also developed in the areas of biomass valorization and bioprocess technology. R & D has established that low-methoxyl pectin stabilizes low-fat set yoghurt and improves their physicochemical properties, rheology, microstructure and sensory liking. Processes for removal of bitterness from kinnow pomace and kinnow pulp residue were also developed.
DBT-National Institute of Animal Biotechnology (DBT-NIAB), Hyderabad has developed a high-density SNP chip using NGS of 43 Indian breeds of cows. This chip is currently under validation using 50 samples of each breed to identify breed specific signatures. NIAB developed a kit for detection of subclinical and clinical mastitis and assessment of microbial quality of milk using magnetic nanoparticles, without the need of any high end equipment . The overall cost to perform one test is less than US $ 0.50/test.
DBT-Regional Centre for Biotechnology (DBT-RCB): RCB has been providing service to academia and industry for testing potential antivirals against SARS-CoV-2 in the cell culture model. So far, over 900 compounds or herbal preparations have been tested. More than 100 students are pursuing doctoral degree programs in Biotechnology, Bioinformatics, and Biostatistics in different RCB laboratories. 25 students are currently registered with RCB for integrated MSc-PhD degree program.
DBT-National Agri-Food Biotechnology Institute (DBT-NABI) has developed a wheat straw polysaccharide derived novel edible coating formulation to prevent the post-harvest shelf-life of perishable fruit crops such as Apple, Peach and Banana. In addition to that, antibody assisted graphene oxide coated gold nanoparticles for rapid bacterial detection and near infrared light enhanced antibacterial activity has been developed which detects food borne pathogens.
DBT- Rajiv Gandhi Centre for Biotechnology (DBT-RGCB): DBT- RGCB has established a nationwide investigator network for research on Head and Neck Cancer ( HNC) alongwith a HNC Bio-repository Utroside-B was identified as a potential anticancer agent against liver Cancer. This compound was licenced to Q-Biomed for further development in a joint initiative with RGCB and OMRF.
DBT-inStem: DBT- inStem was ranked in the top 50 among Indian institutions and 7th in the Life Sciences in India by Nature insex 2020 and has more than thirty publications to its credit since April 2020. InStem scientists have devised probes to enable understanding the many roles of cellular actin which is an important conserved protein from microorganisms to complex organisms and is an emerging drug-target in malaria, leishmaniasis amongst others. Under the aegis of the ADBS programme a recent study examined the consequences of adverse childhood experiences and published a study that has taken an impressive leap forward in modelling Autism – a major and growing public health challenge – in the culture dish. Together, these observations offer new potential therapeutic targets that can be assessed in pre-clinical platforms and eventually lead to more efficient translation to the clinic.
DBT- National Centre for Cell Science (DBT-NCCS): A GMP-Compliant National Repository for banking, safe deposit and supply of characterized mammalian cells for use in Bio-Pharma under BIRAC’s National Biopharma Mission was established in the institute. Promising proteins were identified that could serve as biomarkers and therapeutic targets for multiple myeloma, the second most prevalent blood cancer at the global level.
DBT- Institute of Bioresources and Sustainable Development (DBT-IBSD): Himalayan Bioresources Mission was initiated to connect the East and West, with major emphasis on the development of bio resources of Himalaya and their utilization for future development. In microbial resource programme, microbial repository deposits have increased to more than 72,000 cultures including bacteria, fungi, actinomycetes and yeasts.
DBT-Centre for DNA Fingerprinting and Diagnostics (DBT-CDFD) has provided a) High quality forensic DNA fingerprinting services in 33 cases to the various courts of law, law enforcement and investigative agencies of the country; b) Genetic diagnostic services, prenatal diagnosis, and clinical genetic counselling services to more than 500 people, c) Plant DNA fingerprinting services for 490 samples. 33 research papers have been published and 1 patent was filed.
DBT-National Institute of Plant Genome Research New Delhi: Through marker-assisted selection, large seed characteristic has been transferred into a commercially important desi chickpea variety, JG11 (ICCV 93954). The Improved JG1 variety has a 15-20% increase in yield over the parent. This variety is undergoing Advanced Varietal Trial-2 (AVT-2) at 16 different rainfed areas of India.
Translational Health Science and Technology Institute (THSTI), Faridabad established a dengue virus repository of 40 isolates that are being characterized for vaccine development, dengue structural E protein platform to support antibody-based therapeutic discovery and development and AG129 mouse breeding and colony to facilitate dengue research, Identified a novel HIV-1 Indian clade C native like trimer antigen that has demonstrated favourable immunogenicity in rabbit model, solved the crystal structure of vapB12 antitoxin and generated a hetero-octameric model of vapBC12 toxin-antitoxin complex etc. DBT-THSTI established the following national facilities to support vaccine development:
- Bioassay laboratory was approved by NABL for accreditation under ISO 17025:2017 standard
- Biorepository with over 10 lakh biological samples stored
- Upgradation and expansion of small animal facility
- Construction of ferret facility
- Biosafety level 3 facility at the NCR-Biotech cluster
- Repository of dengue isolates from India
• The institute has published 28 papers, filed 8 patent applications, one copyright application, one trademark application,4 technology developed, 4 technologies transferred, and 1 technology has been commercialized during the year.
DBT- Institute of Life Sciences (DBT-ILS): The institute published 35 research publications during the year 2020. A novel target for regaining sensitivity towards Tamoxifen in case of breast cancer was identified. Human IRGM was shown as a master suppressor of interferon-signaling and targeting IRGM induces a broad antiviral response which can be further developed for anti-viral therapeutics. The Institute of Life Sciences has transferred a technology for production of curcumin sponge for wound healing for commercialization.
DBT-International Center for Genetic Engineering and Biotechnology (DBT-ICGEB): ICGEB scientists during the year were able to raise 4.92 million USD through competitive external project funding and also published 104 publications in peer-reviewed journals. Institute has filed one (1) national and three (3) international Patents and was granted one (1) Patent in 2020.
A link was established between Mtb infection, epigenetics and host immune response, which can be exploited to achieve therapeutic benefits. Using in silico and in vitro screening strategies, potent inhibitors of major cysteine proteases of Plasmodium falciparum, falcipain-2/3, were identified (Bioorg Med Chem. 2020. 28:115155.). Trehalose accumulation was achieved in transgenic rice plants which enabled these plants to tolerate multiple abiotic stresses such as salinity, drought and sodicity and show minimum yield penalty under stress conditions.
DBT- National Institute of Biomedical Genomics (DBT-NIBMG), Kalyani: The ICGC – India Project team of NIBMG participated in the Pan Cancer Analysis of Whole Genomes, a large scale international collaborative initiative of ICGC and TCGA which undertook integrative analysis of 2,658 whole-cancer genomes and their matching normal tissues across 38 tumour types from the Pan-Cancer Analysis of Whole Genomes (PCAWG) Consortium of the International Cancer Genome Consortium (ICGC) and The Cancer Genome Atlas (TCGA). The habit of using RAN/tobacco and GSTP1 AA-genotype together play a significant role in predisposition to oral cancer risk. progress. The institute has published 29 articles, has supported 30 PhD students and 17 iPhD students.
DBT-National Brain Research Institute (DBT-NBRC), Manesar: Japanese Encephalitis virus (JEV) infection was found to induce classical activation (M1) of microglia that drives the production of pro-inflammatory cytokines, while suppressing alternative activation (M2) that could serve to dampen the inflammatory response, thereby inducing inflammatory response, microglial activation, and neuronal apoptosis.Sleep loss, a pathological condition during aging and Alzheimer’s disease, led to impairment of translation at synapses associated with memory storage. DBT- NBRC offers neurological outpatient services at Civil Hospital, Gurgaon to patients from the city as well as neighbouring districts. Patients with epilepsy continue to come to NBRC for sophisticated investigations using the Magnetoencephalography (MEG) facility set up in collaboration with AIIMS, New Delhi. ‘DALI’ a tool in Indian languages for dyslexia assessment in children developed at NBRC remains in high demand and is being increasingly adopted countrywide.
NBRC scientists have developed specialized tools named BRAHMA and ANSH for integrating inputs from neuroimaging data and the clinical information to help in diagnosis of various brain diseases such as Alzheimer’s and Parkinson’s. The tool is standardized on a robust Indian brain template that is representative of the Indian population-specific brain anatomy.
Bharat Immunologicals & Biologicals Corporation Limited (BIBCOL), Bulandshahar, Uttar Pradesh
- BIBCOL continued its production of bivalent oral polio vaccine and supplied bOPV to Ministry of Health and Family Welfare for its polio immunization program.
- For its growth and diversification BIBCOL has taken initiative towards establishment of oral cholera vaccine production under support from BIRAC. BIBCOL has established R&D facility for lab scale production of oral cholera vaccine under technology transfer arrangement with International Vaccine Institute (IVI) Korea.
- BIBCOL aims to produce plasma derived medicines (Albumin and Immunoglobulin) and for this BIBCOL has collaborated with NII New Delhi, and process development at lab scale has been initiated.
Hand sanitizer against COVID - 19
- Four formulations have been developed in R&D stage and license obtained for small scale production.
- BIBCOL has produced and supplied alcohol based formulation to various institutions at affordable rates.
Zinc + Vitamin Tablets were developed and license has been obtained as an immunity booster tablets under FSSAI Act under food supplement category. The formulation consists of Zn, vitamin D3 and Vitamin C as active ingredients as per RDA recommendations. The formulation is being targeted as general immunity enhancement food supplement. The product is also being promoted through e-commerce.
*****
NB/KGS/(DBT inputs)
(रिलीज़ आईडी: 1689929)
|