Ministry of Consumer Affairs, Food & Public Distribution
Bureau of Indian Standards addresses need for reliable, affordable medical assistive technology
Bureau of Indian Standards to prioritize development of standards for 214 critical medical devices; standards to be ready by December, 2025
Posted On:
25 NOV 2024 3:53PM by PIB Delhi
The Bureau of Indian Standards (BIS), India’s National Standards Body is addressing growing need for reliable and affordable medical assistive technology. The Bureau is developing standards for innovative products such as therapeutic footwear, portable ramps, braille displays, and fall detectors, which support individuals with disabilities and enhance their quality of life.
In line with the National Medical Device Policy, 2023, BIS is prioritizing standards development for 214 critical medical devices. These have been identified in consultation with the Department of Pharmaceuticals (DoP). It includes septal closure devices, plasma sterilizers, and phototherapy machines. The initiative is set for phased completion by December 2025.
The Bureau is also driving improvements in healthcare quality, safety, and reliability by developing robust medical device and service standards. By aligning with the Medical Devices Rules, 2017, and the National Medical Device Policy, 2023, BIS is proving to be instrumental in establishing a robust regulatory framework that prioritizes public safety while fostering innovation.
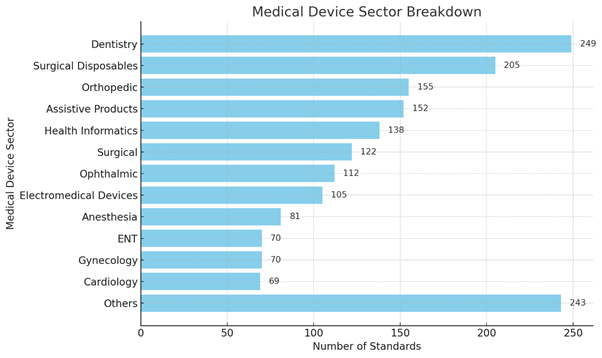
BIS has published over 1,700 standards for the medical sector, covering specialities such as cardiology, neurology, orthopaedics, ophthalmology, and more. Of these, around 1,200 standards specifically focus on medical devices critical to healthcare, including:
- Life-saving devices: Cardiac pacemakers, heart valves, ventilators, and haemodialysis machines.
- Advanced diagnostic tools: X-ray machines, CT scanners, MRI systems, and blood glucose monitors.
- Assistive technology: Hearing aids, wheelchairs, Jaipur Foot, and tactile pathways for visually impaired individuals.
BIS standards in this sector ensure that Indian medical devices are safe, effective, and globally competitive. These efforts build trust among healthcare providers, consumers, and international stakeholders while solidifying India’s reputation as a leader in healthcare innovation.
Key Standards for Medical Devices:
Some of the key Indian Standards developed by BIS include:
- Cardiac Pacemakers: IS 13450 (Part 2/Sec 31): 2021
- Heart Valves: IS 17840
- Hip and Knee Implants: IS 12375
- Ventilators: IS 13450 (Part 2/Sec 12): 2023
- Infant Incubators and Radiant Warmers: IS 13450 (Part 2/Sec 19): 2023 for incubators and IS 13450 (Part 2/Sec 21): 2023 for radiant warmers
- Haemodialysis Machines: IS 13450 (Part 2/Sec 16) : 2019
- Infusion Pumps: IS 13450 (Part 2/Sec 24): 2019
- Advanced Diagnostic Tools:
- X-ray and CT Machines: IS 7620 (Part 1) : 1986
- MRI Systems: IS 13450 (Part 2/Sec 33) : 2018
- Ultrasound Devices: IS 13450 (Part 2/Sec 37): 2019
- ECG: Covered under IS 13450 (Part 2/Sec 25): 2018
- Monitoring Devices:
- Blood Pressure Monitors: IS 13450 (Part 2/Sec 34): 2019
- Blood Glucose Monitors: IS/ISO 15197: 2013
- Pulse Oximeters: IS/ISO 80601-2-61: 2017
***
AD/NS
(Release ID: 2076855)
Visitor Counter : 2127