Ministry of Health and Family Welfare
G20 India Presidency: 3rd HWG Meeting
Three days 3rd G20 Health Working Group Meeting draws to a close
Union Pharma Secretary delivers closing remarks on “Strengthening the global collaboration network on R&D in medical countermeasures”
It is through initiatives like the Global R&D Network that we can collectively build a future where no one is left behind, and access to life-saving medical countermeasures becomes a universal reality: Union Pharma Secretary
“The time is now to build collaboration among nations, institutions, and stakeholders through a global R&D network that fosters innovation and accelerates research”
Emphasizes on the power of collective action and partnerships in expediting research and development as a necessary precursor to equitable distribution, and access to life-saving medical countermeasures against epidemic and endemic diseases
Posted On:
06 JUN 2023 6:07PM by PIB Delhi
“It is through initiatives like the Global R&D Network that we can collectively build a future where no one is left behind, and access to life-saving medical countermeasures becomes a universal reality”. This was stated by Smt S Aparna, Secretary, Dept. of Pharmaceuticals while delivering the closing remarks at the concluding session of the 3rd G20 Health Working Group Meeting, here today. She was joined by Shri Vaidya Rajesh Kotecha, Secretary, Ministry of AYUSH and Dr Rajiv Bahl, Secretary, Dept of Health Research and DG, ICMR.
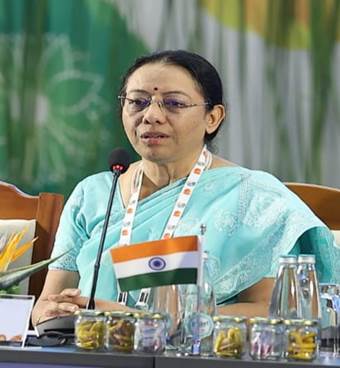
Addressing the session, Smt S Aparna expressed her appreciation towards all the delegates from G20 member states, invited countries, and International Organizations for contributing their valuable insights on the establishment of a global R&D network within the Global Medical Countermeasures Platform to support Pandemic Preparedness and Response. She stated that “the discussions have helped to define the pathway for collaborative partnerships and provided us with a framework to envision the global R&D network.”
Underscoring the impact of the COVID-19 pandemic on the healthcare systems around the world, the Union Pharma Secretary said that “the time is now to build collaboration among nations, institutions, and stakeholders through a global R&D network that fosters innovation and accelerates research. This would be a necessary element to build the requisite agility and bench strength on a global scale to predict, prepare for and respond to future health emergencies in a robust, equitable and timely manner”.
She emphasized on “the power of collective action and partnerships in expediting research and development, as a necessary precursor to equitable distribution, and access to life-saving medical countermeasures against epidemic and endemic diseases.” “The timely deployment of appropriate countermeasures in an emergency, also relies to a large extent, on those having been developed and tested by continuous interaction with a variety of stakeholders in different locations and socio-economic situations during peace time”, she further stated.

Noting that deliberations over the course of the last three days have delved into the fundamental principles and components required to establish a thriving network that enhances capabilities for early stage and preparatory research, and ensures universal access to effective and affordable medical countermeasures, she stated that “key aspects such as leveraging the respective strengths of partners, structured knowledge sharing, prioritization, resource allocation, capacity building, and effective technology transfer have been identified as essential pillars of a well-functioning global R&D network.”
On the structure of such a mechanism, Smt. S Aparna stated that “a Network of Networks which encouraging regional and local cooperation as well as greater alignment of existing partnerships and principles such as those found in World Health Organization (WHO) Blueprint for epidemics and the 100 days Mission would be an efficient and effective path towards Impact driven Collaboration”. “That such a collaboration on R&D should factor in a globally accessible database covering priority pathogens, ongoing research on vaccines, therapeutics and diagnostics (VTDs), and development of disease agnostic technologies would help to address challenges such as the asymmetry of information, the lack of availability of critical materials, low attention to certain products needed by small patient populations as well as the inequitable access to potential solutions that currently obtains across geographies and communities”, she stated.
A fieldtrip was organized for the G20 delegates to visit the Genome Valley which is India’s first organized cluster for Life Sciences R&D located in Hyderabad. One of the key features of Genome Valley is the presence of several research and innovation institutions. The G20 delegates visited the Bharat Biotech International and National Animal Resource Facility for Biomedical Research (NARFBR), Indian Council of Medical Research (ICMR) facilities. The delegates visited the facilities of these institutions, and garnered in-depth knowledge of the inception of Covaxin, India’s indigenous Covid-19 vaccine by Bharat Biotech International that was developed in collaboration with the Indian Council of Medical Research (ICMR) – National Institute of Virology (NIV). The G20 delegates were also provided tours of the institutions, visiting the facilities housed in the institutions, and engaged with the authorities, sharing their perspectives and queries.

Day 3 of the HWG meeting also witnessed a panel discussion on “Envisaging a Global R&D Network for Research in VTDs to enhance PPR”. Dr. Christopher Elias, President (Global Development), Bill & Melinda Gates Foundation stated the need to unlock the power of innovation and R&D for a healthier present and future. Dr Pushpa Vijay Raghavan, Board Member, Medicines Patent Pool shared her experiences in developing manufacturing networks for therapeutics and its implications on vaccines, therapeutics and diagnostics on future pandemics. Dr. Kavita Singh, Director (South Asia), Drugs for Neglected Diseases Initiative (DNDi) highlighted the importance of partnerships for creating a global research and development network.
Dr Rajiv Bahl, Secretary elaborated on the benefits of global MCM coordination. He stated that “global VTD ecosystem of medical countermeasures coordination platform augmented with VTDs R&D and Manufacturing Networks is identified to be critical for reducing existing inequities in availability and accessibility of safe, effective, quality and affordable VTDs”.
Shri Vaidya Rajesh Kotecha highlighted the need for translational research in AYUSH to supplement the overall MCM platform. He emphasized on the need for exploration of new leads in traditional medicine, fostering collaboration and adoption of multidisciplinary approach towards ensuring cost-effective, safe and holistic healthcare.

Shri Lav Agarwal, Additional Secretary, Ministry of Health and Family Welfare; Mr. Satish Reddy, Chairman, Dr Reddy’s Laboratories; Mr Pankaj Patel, Chairman, Zydus Life Sciences; representatives from the G20 member countries, special invitee countries, International Organizations, forums and partners like WHO, World Bank, WEF etc., and senior officers of the Union Government were present.
****
MV
HFW/HFM-G20 HWG Side Event Concludes/06thJune2023/1
(Release ID: 1930273)
Visitor Counter : 1963