Ministry of Science & Technology
Newly identified property of metallo-nanozymes could transform bioenergy and therapeutic applications
प्रविष्टि तिथि:
08 APR 2025 5:39PM by PIB Delhi
Scientists have found that metallo-nanozymes, or artificial biocatalysts, mimicking natural enzymes that utilize metal ions for their catalytic activity are capable of controlling electron transfer, a crucial process for regulating cellular energy. This newly identified property of metallo-nanozymes seamlessly integrate into biological systems, enabling sustainable energy production, medical inventions, and environmental solutions.
While nanozymes are gaining attention, current-generation nanozymes pose several challenges, particularly in therapeutic applications. Many nanozymes lack well-defined active sites that can lead to uncontrolled electron transfer and unwanted side reactions.
The unregulated electron transfer rates can lead to leakage of electrons, generating toxic reactive oxygen species (ROS) and affecting ATP production, which may result in cellular dysfunction and related complications.
To overcome this challenge, next-generation nanozymes must be designed with carefully engineered active sites that precisely regulate interactions with substrates and control electron flow.
Dr. Amit Vernekar, an INSPIRE Faculty fellow and his Ph.D. student, Adarsh Fatrekar, at CSIR-Central Leather Research Institute (CLRI), Chennai, have delineated the role of Cu-Phen, a self-assembled nanozyme designed with a catalyst-by-design strategy.
Composed of amino acid phenylalanine ligands coordinated to copper ions (Cu²⁺) and with an assembled nano-structure, Cu-Phen represents a significant advancement in artificial enzyme development. Unlike other nanozymes with undefined and open active sites, Cu-Phen features a careful design with a well-defined active site that ensures precise electron transfer, mimicking the functions of natural enzymes involved in the energy pathways of cells.
Cu-Phen operates by interacting with cytochrome c, a critical electron donor protein in the electron transport chain, in a receptor-ligand manner similar to that observed in natural systems. Cu-Phen induces specific hydrophobic interactions with cytochrome c and followed by it traverses a unique proton-coupled electron transfer to the Cu2+ centre.
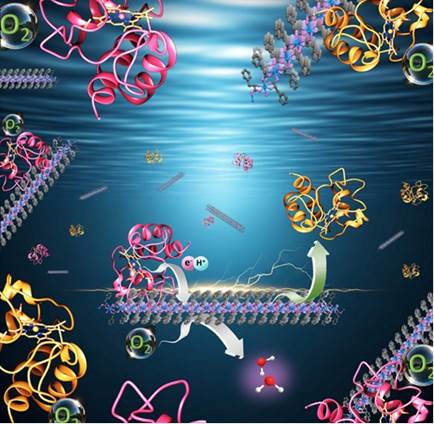
Fig: An illustration depicting the interaction between the mitochondrial protein cytochrome c and Cu-Phen, highlighting electron transfer for oxygen reduction to water.
This phenomenon efficiently reduces oxygen to water, avoiding the generation of harmful byproducts, like ROS, that can damage cells and lead to oxidative stress and other diseases.
These findings could have major implications for regulated bioenergy, where controlling electron flow is vital for cellular energy production. As nanozymes are gaining traction in several applications, this work could also help pave the way for designing more advanced artificial enzymes for biotechnology and energy research.
The study, recently published in Journal of Materials Chemistry A, highlights how Cu-Phen’s structure assists controlled electron transfer, setting it apart from other nanozymes.
The work also opens exciting new possibilities for the future of nanozyme engineering, where precision in active site design and electron flow regulation are crucial for harnessing the full potential of artificial enzymes in bio-inspired applications. With these advancements, scientists are pushing the limits for developing smarter and more efficient enzyme-like catalysts that can seamlessly integrate into biological systems, enabling sustainable energy production, medical inventions, and environmental solutions.
***
NKR/PSM
(रिलीज़ आईडी: 2120104)
आगंतुक पटल : 1905