Ministry of Science & Technology
New cathode material can produce high-performance, cost-effective, environment-friendly Na-ion batteries as next-generation energy storage systems
प्रविष्टि तिथि:
16 MAY 2023 4:20PM by PIB Delhi
Scientists have found an avenue that can simultaneously address the air/water-instability and structural-cum-electrochemical instability of Sodium–transition-metal–oxide-based cathode materials for Sodium-ion batteries and, accordingly, have developed new air/water-stable stable and high-performance cathode materials. The newly developed materials exhibit high electrochemical cyclic stability and stability upon exposure to air/water, thus, facilitating the development of systems that are expected to serve as cost-effective and sustainable energy storage systems for a range of applications, including consumer electronic devices, grid energy storage, storage of energy harvested from renewables and, eventually, electric vehicles.
As the importance of battery-driven electric vehicles increases due to climate and environmental concerns, development of a cost-effective, resource-friendly, safe, and sustainable alkali metal-ion battery system beyond the Li-ion system is essential. India has an abundance of Na-sources, which renders the upcoming Na-ion battery system extremely important in the Indian context. Like any alkali metal-ion battery cell, a Na-ion cell has cathode and anode active materials (supported on metallic current collector foils), which facilitate reversible insertion/removal of the charge carrier (viz., Na-ion) during charge/discharge of the cell. In such a cell, the cathode material initiates as the Na-reservoir, with the cell performances depending on the structural/electrochemical stability of the electrodes, Na-transport kinetics, and various resistances (which are dynamic in nature).
Despite the many advantages of Sodium-ion batteries, the electrochemical behaviour/performances of the ‘layered’ Na-TM-oxide-based cathode materials and their stability upon moisture exposure need substantial improvements for widespread development and usage of Na-ion battery systems for a variety of applications to be a reality. This is because such lack of stability renders handling/storage of Na-TM-oxides challenging and also negatively affects their electrochemical performance. Besides, the water instability mandates the usage of toxic-hazardous-expensive chemicals like N-Methyl-2-pyrrolidone (NMP) for electrode preparation, as opposed to the possible usage of water-based slurries.
Prof Amartya Mukhopadhyay’s group at IIT Bombay, in their recent research, exploited materials science and electrochemical principles to reveal the dominant factors and controlling parameters that can help develop high-performance Na-ion batteries in order to address this challenge. In their research supported by the Science and Engineering Research Board (SERB), an attached institution of the Department of Science and Technology (DST) and DST’s Materials for Energy Storage scheme, they have evolved a universal design criterion, paving the way towards successful design and widespread development of environmentally stable and high-performance cathodes for the sustainable Na-ion battery system and beyond. The researchers have suggested a change in the alternate slab layered structure of the Na-TM-oxide structure of the Na-ion battery cathode by introducing “interslab” spacing by tuning the TM-O bond covalency in a paper published in the journal Chemical Communications.
The typical ‘layered’ Na-TM-oxide structure is built of alternate ‘slabs’ composed of NaO2 (O-Na-O) and TMO2 (O-TM-O), with the O-ions (which bear a net negative charge) being shared by the TM-ions and the Na-ions in their respective layers (the O-ion is common to both the cations). Here, while the TM-O bond is iono-covalent in nature, the Na-O is predominantly ionic. In such a ‘layered’ structure, tuning the degree of covalency of the TM-O bond by designing a suitable combination of cations in the TM-layer can tune the net/effective negative charge on the O-ion, which, in turn, can affect the electrostatic attraction between the Na- and O-ions and also the repulsions between the O-ions across the Na-layer.
For example, a higher net/effective negative charge on the O-ion due to reduced TM-O covalency will cause the accrued electrostatic attraction between the Na-ions and O-ions, rendering the predominantly ionic Na-O bond stronger and shorter. In the study published in the journal Adv. Energy Mater, the researchers showed that the above should lead to a reduced ‘inter-slab’ spacing (or Na-layer thickness) and can improve the air/water stability, as well as suppress the occurrence of deleterious structural/phase transitions in the O3-structured Na-TM-oxide based cathode materials. This can help in the development of high-performance water-stable cathodes for Na-ion batteries which can be prepared via health/environment-friendly and cost-effective ‘aqueous’ processing route (as also demonstrated in another publication in J. Mater. Chem. A).
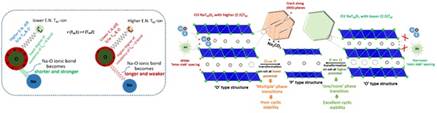
Figure 1. Depiction of the influence of TM-O bond covalency on the character of the Na-O bond and, in turn, the Na-layer thickness (i.e., ‘inter-slab’ spacing) in a ‘layered’ Na-TM-oxide structure. TM: transition metal, E.N.: electronegativity; for right-hand-side figure, grey balls: Na-ions, green balls: O-ions, blue parallelograms: TMO6 octahedra (as published in, Adv. Energy Mater. (2023) 2204407; DOI: 10.1002/aenm.202204407).
By contrast, a lower effective negative charge on the O-ion, as induced by greater TM-O bond covalency, would decrease the electrostatic attraction between the Na- and O-ions, resulting in a weaker-cum-longer Na-O bond and, thus, an enlarged ‘inter-slab’ spacing (Na-layer thickness).
This will facilitate faster Na-transport kinetics and, thus, enhanced rate-capability of the cathode, promising to enhance the power density of Na-ion batteries significantly, as demonstrated in the study published in the journal Chem. Commun.. Using an allied logic, i.e., increase in the TM-O bond covalency to lessen the effective negative charge on O-ions, as is needed to stabilize the prismatic O-coordination of Na-ions (as in a certain structure types, i.e., P2), the group has been able to stabilize the inherently higher rate-capable and electrochemically more stable P2 structure for Na-TM-oxide cathode materials at a considerably higher starting Na-content of ~0.84 p.f.u. (as compared to the typically obtained < 0.7 p.f.u.); leading to significantly enhanced Na-storage capacity and also air/water-stability. This has been reported in the journal Chem. Mater. The above developments are of immense practical significance, and the associated new learnings are expected to facilitate widespread development of high-performance and cost-effective Na-ion battery systems through health/environment-friendly processing of electrodes.
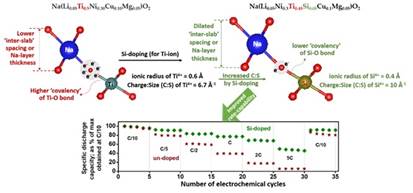
Figure 2. Depiction of the influence of raised TM-O bond covalency on the widening of the ‘inter-slab’ spacing (i.e., Na-layer thickness) and, thus, enhancement of the rate-capability, as presented for the newly-developed high rate-capable O3-structured Na(Li0.05Ni0.3Ti0.45Si0.05Cu0.1Mg0.05)O2 cathode material (as published in, Chem. Commun. 59 (2023) 4332-4335).

Figure 3. Depiction of the influence of raised TM-O bond covalency on the stabilization of the P2 structure for Na-TM-oxide even at a high Na-content of ~0.84 p.f.u.; thus, leading to the development of a high capacity (viz., greater than most reported to-date) and very stable P2-structured Na([]0.06Li0.04Mg0.02Ni0.22Mn0.66)O2 cathode material, which exhibits excellent performances in Na-ion cells (as published in, Chem. Mater. 34[23] (2022) 10470–10483).
Recent publication links
https://doi.org/10.1002/aenm.202204407
https://pubs.rsc.org/en/content/articlelanding/2023/cc/d3cc00079f
https://pubs.acs.org/doi/full/10.1021/acs.chemmater.2c02478
https://pubs.rsc.org/en/content/articlehtml/2020/ta/d0ta05169a
The associated patents
I. Biswas, B. S. Kumar, A. Pradeep, and A. Mukhopadhyay: An O3-structured ‘Layered’ Sodium Transition Metal Oxide-based Cathode Material for Na-ion batteries; Indian Patent Application No.: 202321016587, dated 13-03-2023 (Patent filed)
B. S. Kumar, A. Pradeep, Amardeep and A. Mukhopadhyay: High Sodium Containing P2-type Sodium Transition Metal Oxide-based Cathode for Na-ion Batteries; Indian Patent No.: 406595; dated: 14/09/2022 (Patent granted)
<><><><>
SNC/PK
(रिलीज़ आईडी: 1924518)
आगंतुक पटल : 2633