Ministry of Science & Technology
A tug of war in gold nanoscale assembly can help produce better biosensors
प्रविष्टि तिथि:
12 SEP 2025 3:57PM by PIB Delhi
Scientists have uncovered how gold nanoparticles—the tiny particles that give rise to powerful optical technologies—change their behaviour when exposed to certain every day molecules like Amino Acids & Salts, paving the way towards smarter biosensors, better diagnostic tools, and even more reliable drug delivery systems.
Gold nanoparticles are special because they interact with light in unique ways. Their colour and optical response depend on whether they are alone or clustered together. When they aggregate, their optical properties modulate, which is why they are widely used in biosensors and imaging. But this property is a double-edged sword—uncontrolled aggregation can make these systems unreliable. Understanding how to control this process has been a major goal for scientists.
Scientists from S N Bose National Centre for Basic Sciences, an autonomous institution of the Department of Science and Technology (DST) found a way to control this aggregation or clustering.
A team led by Prof. Manik Pradhan, introduced two molecules -- Guanidine Hydrochloride (GdnHCl) – a powerful salt known for breaking proteins apart in laboratories & L-Tryptophan (L-Trp) – an amino acid we consume daily, found in proteins and often associated with sleep and relaxation.
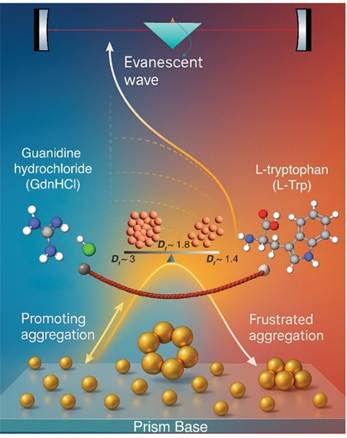
Figure 1: The graphic shows real-time monitoring of gold nanoparticle aggregation using EW-CRDS, where the evanescent wave probes GdnHCl-induced aggregation and its alteration by L-Trp, resulting in frustrated aggregation.
They found that When GdnHCl was added, gold nanoparticles quickly lost their repulsion and clumped into dense, compact clusters. But when L-Trp entered the scene alongside GdnHCl, the story changed. Instead of tight clumps, the nanoparticles formed looser, branched networks.
The researchers called this “frustrated aggregation”—as if the nanoparticles wanted to clump tightly, but the amino acid kept interfering.
The team comprising Soumyadipta Chakraborty, Dr. Jayeta Banerjee, Indrayani Patra, Dr. Puspendu Barik, and Professor Manik Pradhan employed a highly sensitive optical technique a cutting-edge method called Evanescent Wave Cavity Ringdown Spectroscopy (EW-CRDS) to study this. They found that L-Trp stabilizes the guanidinium ions, softening their effect. This leads to slower aggregation and a new, open structure.
EW-CRDS opens up an entirely new window into studying delicate processes at surfaces with unmatched sensitivity.
This pioneering study, highlighted in the Journal Analytical Chemistry, not only answers fundamental questions in nanoscience but also pushes the boundaries of how we study light and matter.
***
NKR/PSM
(रिलीज़ आईडी: 2165986)
आगंतुक पटल : 261