Ministry of Science & Technology
Single-molecule techniques reveal new dynamics of protein folding associated with Alzheimer’s disease progression
Posted On:
12 JUL 2024 6:09PM by PIB Delhi
A new way to study protein folding & associated chaperons that saves proteins from non-native interaction, could help understand what exactly triggers the folding. The understanding could help track progression of diseases like cancer, Parkinsons and Alzheimer’s.
Proteins participate in almost every process within the cell. They need to adapt a well defined 3-D structure in order to carry out their assigned function called ‘native conformation’. But due to several chemical, environmental or physical stress conditions, the protein molecules may get misfolded or unfolded, leading to disfunction. It results in aggregation of toxic material in the cell, resulting in diseases. Neuro-degenerative diseases such as Alzheimer’s or Parkinson’s Disease have been linked to formation of toxic aggregates within a cell.
While several newly translated proteins can fold spontaneously many of them require the assistance of molecular chaperones to achieve their native state and to avoid non-native interactions. Molecular chaperones are essential for keeping the protein molecules functioning properly. In addition to helping with folding, they can repair unfolding and misfolding too.
In light of the importance of molecular chaperones in our well-being, researchers been studying their structure and functioning within the cell. Bulk biochemical measurements have informed us on the protein folding efficiency and prevention of aggregation when chaperones are present during protein folding.
However, conventional bulk experiments cannot probe into the heterogeneity of the chaperone molecules and how each of these molecules function in diverse cells. Also, the short-lived transient states do not come under the scanner of bulk experiments. The significance of these transient states in the metabolic processes remains little understood.
In recent years, development of single-molecule techniques has opened new avenues to explore the fundamental properties of biomolecules involved in diverse biochemical reactions.
A team at the S.N. Bose National Centre for Basic Sciences (an autonomous institute of DST) under Prof. Shubhasis Halder, is using a Covalent Magnetic Tweezer (CMT) fabricated in their lab, to study physical and chemical properties of protein molecules and action of chaperones on how these molecules fold and function.
This innovative approach has provided unprecedented insights into the intricate dynamics of chaperone-assisted protein folding. Among the key players in this molecular ballet are the heat shock proteins Hsp70 and Hsp90, two of the most studied molecular chaperones.
Single-molecule force spectroscopy has revealed the intricate dynamics of Hsp70-induced protein manipulation. The intricate details are crucial for understanding how Hsp70 assists in protein folding, stabilization, and transport under various cellular conditions.
Hsp90 is also an important chaperone known to activate and stabilize many proteins, including steroid hormone receptors and signaling kinases. Single-molecule techniques were used to characterize the multiple pathways and states of the Hsp90 complex. This research, unveiled the multifaceted capabilities of magnetic tweezers in manipulating protein structures.
Their findings have revealed novel mechanisms of molecular chaperones and provided insights into their functioning and implications for protein homeostasis and human diseases. A review of the series of studies was published in the journal Trends in Biochem Sciences.
They highlight the mechanical dynamics underlying chaperone interactions with substrates under force, and that chaperones, particularly those localized within cellular tunnels, harness the force generated during protein translocation to facilitate substrate folding.
These tunnel-associated chaperones utilize the mechanical energy derived from the tunnel to guide the folding process, thereby ensuring the proper maturation of proteins crucial for vital cellular functions. Furthermore, the investigation unveils the diverse mechanical functions exhibited by chaperones under force.
The researchers are now beginning to understand the exact mechanism of how Alzheimer's sets in due to brain stiffness. When the physical basis of degenerative diseases like Alzheimer’s and Parkinson’s Disease are understood at a molecular level, drugs can be designed to target the mechanical roles of the chaperones. That will make it easier to prevent the progression of these diseases.
“However, a lot remains unanswered as we work at the junction of basic and translational research”, says Debojyoti Chowdhury, the co-author of the review paper.
Once existing gaps in understanding of dynamics of chaperones and their client proteins is understood, pharmaceutical science will be poised for the next leap forward. Single-molecule techniques hold the key to this revolution.
Related publications:
Ayush Chandrakant Mistry et al, Elucidating Novel Mechanisms of Molecular Chaperones by Single-molecule Technologies, Trends in Biochemical Sciences, 2023
Additional reference
Lene Clausen et al, Molecular Chaperones in Human Disorders, Advances in Protein Chemistry and Structural Biology, 2019
Chaudhuri et al., Direct Observation of the Mechanical Role of Bacterial Chaperones in Protein Folding, ACS Biomacromolecules, 2022
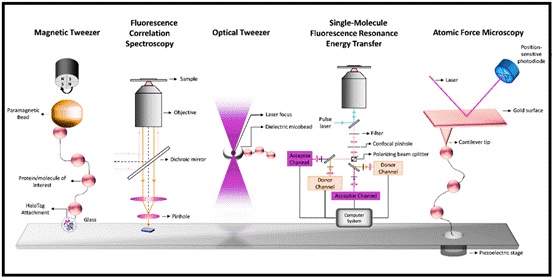
Fig 1: Single-molecule techniques: Magnetic tweezer, Fluorescence correlation spectroscopy, Optical tweezer, Single-molecule fluorescence resonance energy transfer (FRET):Atomic force microscopy.
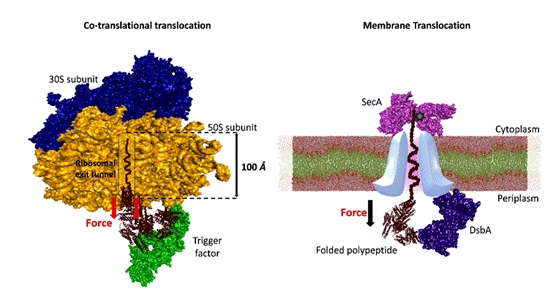
Fig 2. Mechanical folding of substrate proteins by tunnel-associated chaperones (Here, trigger factor and DsbA).
*****
PK/PSM
(Release ID: 2032814)
Visitor Counter : 2041