PIB Headquarters
PIB’S BULLETIN ON COVID-19
Posted On:
07 DEC 2021 6:08PM by PIB Delhi


- 128.76 cr vaccine doses have been administered so far under Nationwide Vaccination Drive
- India's Active caseload currently stands at 95,014; lowest in 554 days
- Active cases account for less than 1% of total cases, currently at 0.27%; Lowest since March 2020
- Recovery Rate currently at 98.36%; Highest since March 2020
- 10,004 recoveries in the last 24 hours increases Total Recoveries to 3,40,79,612
- 6,822 new cases in the last 24 hours; lowest in 558 days
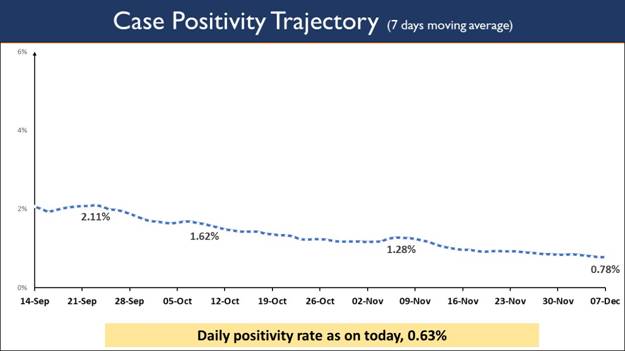
- Daily positivity rate (0.63%) less than 2% for last 64 days
- Weekly Positivity Rate (0.78%) less than 1% for last 23 days
- 64.94 cr Total Tests conducted so far
|
#Unite2FightCorona #IndiaFightsCorona
PRESS INFORMATION BUREAU
MINISTRY OF INFORMATION & BROADCASTING
GOVERNMENT OF INDIA
*****

COVID-19 Update
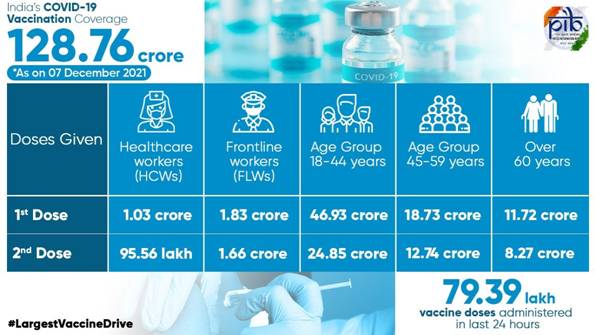
India’s Cumulative COVID-19 Vaccination Coverage exceeds128.76 Cr
More than 79 Lakh doses administered in the last 24 hours
Recovery Rate currently at 98.36%
6,822 New Cases reported in the last 24 hours is lowest in 558 days
India's Active Caseload (95,014) is lowest in 554 days
Weekly Positivity Rate (0.78%) less than 1% for last23 days
With the administration of 79,39,038 vaccine doses in the last 24 hours, India’s COVID-19 vaccination coverage has exceeded 128.76 Cr (1,28,76,10,590) as per provisional reports till 7 am today. This has been achieved through 1,34,23,668 sessions.
The break-up of the cumulative figureas per the provisional report till 7 am today include:
|
HCWs
|
1st Dose
|
1,03,84,773
|
|
2nd Dose
|
95,56,046
|
|
FLWs
|
1st Dose
|
1,83,81,553
|
|
2nd Dose
|
1,66,08,872
|
|
Age Group 18-44 years
|
1st Dose
|
46,93,17,106
|
|
2nd Dose
|
24,85,78,165
|
|
Age Group 45-59 years
|
1st Dose
|
18,73,53,131
|
|
2nd Dose
|
12,74,17,445
|
|
Over 60 years
|
1st Dose
|
11,72,45,359
|
|
2nd Dose
|
8,27,68,140
|
|
Total
|
1,28,76,10,590
|
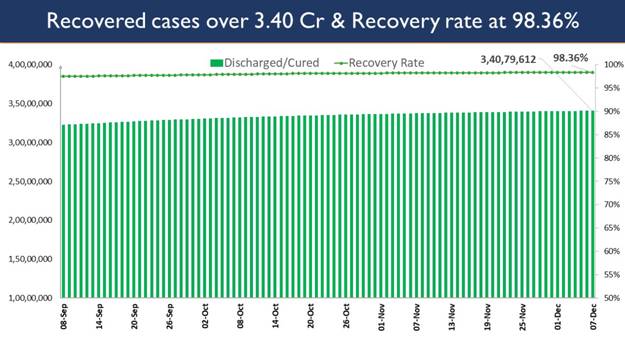
The recovery of 10,004 patients in the last 24 hours has increased the cumulative tally of recovered patients (since the beginning of the pandemic) to 3,40,79,612.
Consequently, India’s recovery rate stands at 98.36%,highest since March 2020.
Sustained and collaborative efforts by the Centre and the States/UTs continue the trend of less than 50,000 Daily New Cases that is being reported for 163 consecutive days now.
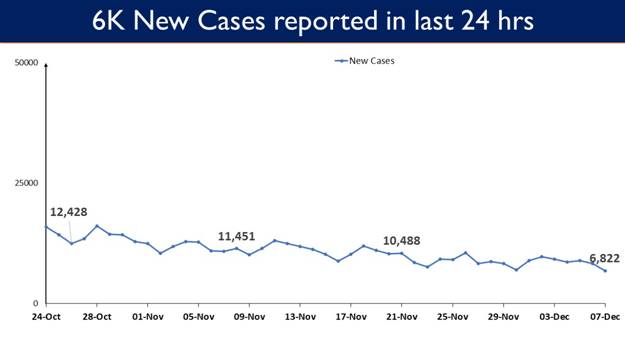
6,822 new cases were reported in the last 24 hours. This is lowest in 558 days.
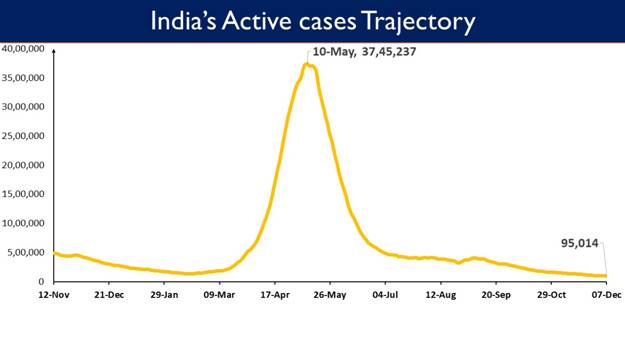
India’s Active Caseload presentlyat 95,014 is lowest in 554 days.Active cases constitute 0.27% of the country's total Positive Cases, which is lowest since March 2020.
The testing capacity across the country continues to be expanded. The last 24 hours saw a total of 10,79,384tests being conducted. India has so far conducted over 64.94 Cr (64,94,47,014) cumulative tests.
While testing capacity has been enhanced across the country, Weekly Positivity Rate at 0.78% remains less than 1% for the last 23 days now. The Daily Positivity rate reported to be 0.63%. The daily Positivity rate has remained below 2% for last 64 days and below 3% for 99 consecutive days now.
https://pib.gov.in/PressReleasePage.aspx?PRID=1778689
Update on COVID-19 Vaccine Availability in States/UTs
More than 139 Crore vaccine doses provided to States/UTs
More than 20.13 Crore balance and unutilized vaccine doses still available with States/UTs
The Union Government is committed to accelerating the pace and expanding the scope of COVID-19 vaccination throughout the country. The nationwide COVID 19 vaccination started on 16th January 2021. The new phase of universalization of COVID-19 vaccination commenced from 21st June 2021. The vaccination drive has been ramped up through availability of more vaccines, advance visibility of vaccine availability to States and UTs for enabling better planning by them, and streamlining the vaccine supply chain.
As part of the nationwide vaccination drive, Government of India has been supporting the States and UTs by providing them COVID Vaccines free of cost. In the new phase of the universalization of the COVID19 vaccination drive, the Union Government will procure and supply (free of cost) 75% of the vaccines being produced by the vaccinemanufacturers in the country to States and UTs.
|
VACCINE DOSES
|
(As on 7th December, 2021)
|
|
SUPPLIED
|
1,39,06,60,790
|
|
BALANCE AVAILABLE
|
20,13,38,526
|
More than 139 crore (1,39,06,60,790) vaccine doses have been provided to States/UTs so far through Govt of India (free of cost channel) and through direct state procurement category.
More than 20.13Cr (20,13,38,526) balance and unutilized COVID Vaccine doses are still available with the States/UTs to be administered.
https://pib.gov.in/PressReleasePage.aspx?PRID=1778680
Special Training to Doctors and Para-Medical Staff in Handling Various Types of Epidemics
The Government has taken several measures to enable training and capacity building in COVID-19. Training, including virtual training, has been provided to doctors, nurses and allied healthcare professionals and volunteers regarding the relevant aspects of COVID-19. The Government of India has utilized the iGOT (Integrated Government Online Training) platform to train various personnel. Since the beginning of COVID-19, close to 14 lakh unique users across the country have registered on this platform, recording enrollment of 29.29 lakh for different course. Additionally, more than 80 lakh health workforces have been trained in COVID related topics through the State Governments. Webinars and tutorials on various COVID related subjects were also uploaded by the Ministry of Health and Family Welfare and reputed institutes like AIIMS (New Delhi), NIMHANS, PGIMER, JIPMER, etc. that recorded a viewership of 2.23 crore.
As informed by National Medical Commission (NMC), a competency-based module was introduced by the erstwhile Board of Governors,Medical Council of India (BoG-MCI) on management of pandemics in the MBBS curriculum in August, 2020.
The Union Minister of State for Health and Family Welfare, Dr Bharati Pravin Pawar stated this in a written reply in the Rajya Sabha today.
https://pib.gov.in/PressReleasePage.aspx?PRID=1778830
Measures Taken to Minimize Threat of Any Resurgence of Covid-19 Pandemic
Government of India continues to keep a close watch over COVID-19 situation in the country by strict observance of fivefold strategy of test-track-treat, Covid Appropriate behaviour and vaccination against COVID-19 to prevent and mitigate the impact of any resurgence of COVID-19 trajectory in the country.
Government of India has also established an Indian SARS-CoV-2 Genomic Surveillance Consortium (INSACOG) for genomic sequencing and tracking the evolution of variant strains of SARS-CoV-2.
Support is provided to States/UTs to enhance preparedness and response capacities against COVID-19 and other public health emergencies. Various initiatives have been taken by the Government to provide technical guidance and further strengthen health infrastructure, availability of essential logistics including drugs and medical oxygen supply to manage COVID-19. Some of the major areas of intervention include:
- More than 150 guidelines/advisories/SoPs/plans have been provided to States/UTs.
- Guidelines on Clinical management of COVID-19 with emerging scientific evidence widely circulated.
- Guidelines for management of COVID-19 in children on acute presentation of COVID-19 as well as Multisystem Inflammatory Syndrome (MIS-C) in children and adolescents found temporally related to COVID-19 have been issued.
- The number of testing labs for detection of Covid-19 has been increased to 3062 labs as on 1st December 2021.
- Guidelines and checklists on prevention and clinical management of Mucormycosis disseminated to all States/UTs.
- A Comprehensive Guidelines for Management of Post-Covid Sequel was issued by MoHFW after expert consultations to guide doctors on post-COVID-19 complications and their management.
Adequate doses to vaccinate all eligible 1st dose and due 2nd dose beneficiaries aged 18 years and above by December 2021 are made available to States/UTs.
The Union Minister of State for Health and Family Welfare, Dr Bharati Pravin Pawar stated this in a written reply in the Rajya Sabha today.
https://pib.gov.in/PressReleasePage.aspx?PRID=1778832
Export of Covid-19 Vaccines
India supplied COVID-19 related medical and other assistance, to over 150 countries since the beginning of COVID-19 pandemic.
Since the start of Vaccine Maitri Programme in January, 2021, India has supplied 723.435 lakh doses of COVID vaccine to 94 countries and 2 UN entities in the form of grant, commercial export or through COVAX till 29th November, 2021.
In the fight against the COVID-19 pandemic during the second wave, support in the form of COVID related equipment and medicines were received from more than 50 countries. These included supplies from foreign governments, private companies, Indian associations abroad, etc.
The Union Minister of State for Health and Family Welfare, Dr Bharati Pravin Pawar stated this in a written reply in the Rajya Sabha today.
https://pib.gov.in/PressReleasePage.aspx?PRID=1778837
Vaccination Program for Children and Under-18 Population
National Expert Group on Vaccine Administration for Covid (NEGVAC) and National Technical Advisory Group on Immunization (NTAGI) are deliberating and considering scientific evidences related to vaccination of beneficiaries aged less than 18 years.
Government of India has been supplying Covid-19 vaccines free of cost to the States/UTs for administration to prioritized beneficiaries as recommended by NEGVAC.
ZyCoV-D vaccine manufactured by M/s Cadila Healthcare has received the approval for Restricted Use in Emergency Setting by the National Regulator i.e., Drug Controller General of India (DCGI) for the age group of 12 years and above based on the interim clinical data of Phase II &Phase III clinical trial conducted in the country.
Following COVID-19 vaccines are under clinical trials in the country for age-group of less than 18 years:
- M/s Bharat Biotech is conducting Phase II/III clinical trial of COVAXIN on Healthy Volunteers aged 2 to 18 years & firm has submitted interim safety & immunogenicity data to the National Regulator.
- M/s Serum institute of India is conducting Phase II/III clinical trial of Nanoparticle Vaccine (Liquid) (COVOVAX) in 920 subjects of >2 to 17 years age group.
- M/s Biological E Ltd., is conducting Phase II/III clinical trial of RBD of SARS-CoV-2 gene in 624 subjects of ≥5 to <18years age group.
- M/s Johnson & Johnson Pvt. Ltd., is conducting Phase II/III clinical trial of Ad.26COV.2S vaccine in age group of 12-17 years (Global Clinical trial wherein India is one of the clinical trial sites).
The approval of aforementioned COVID-19 vaccines is dependent on the successful completion of clinical trials and submission of requisite data to the National Regulator i.e., Drug Controller General of India as per the requirements of New Drugs and Clinical Trials Rules, 2019 under Drugs and Cosmetics Act, 1940.
Inclusion of Diseases like Covid-19 and Dengue under AB-PMJAY
Treatment of diseases like COVID-19 and dengue is included under Ayushman Bharat –Pradhan Mantri Jan Arogya Yojana (AB-PMJAY). Details of specific Health Benefit Packages for COVID-19 and dengue are at Annexure-I. State-wise details of COVID-19 testing and treatment under AB-PMJAY are at Annexure-II.
Annexure-I
Details of specific Health Benefit Packages for COVID-19 under AB-PMJAY:
|
Sl. No.
|
Package Name
|
Procedure Name
|
|
1
|
Laboratory Tests for COVID-19 Infection (PCR) (Reimbursement level for this package will be as per the ICMR guidelines, issued from time to time)
|
Laboratory Tests for COVID-19 Infection (PCR) (Reimbursement level for this package will be as per the ICMR guidelines, issued from time to time)
|
|
2
|
Laboratory Tests for COVID-19 Infection (PCR) (Reimbursement level for this package will be as per the ICMR guidelines, issued from time to time)
|
Laboratory Tests for COVID-19 Infection (PCR) (Reimbursement level for this package will be as per the ICMR guidelines, issued from time to time)
|
|
3
|
Treatment of COVID-19 Infection
|
Treatment of COVID-19 Infection
|
Details of specific Health Benefit Packages for dengue under AB-PMJAY:
|
Sl. No.
|
Package Name
|
Procedure Name
|
|
1
|
Dengue Fever
|
Dengue Fever
|
|
2
|
Dengue Fever
|
Dengue Hemorrhagic Fever
|
|
3
|
Dengue Fever
|
Dengue Shock Syndrome
|
Annexure-II
COVID-19 Pre-authorization raised under AB-PMJAY:
|
State/UT
|
Testing Count
|
Treatment Count
|
|
Andaman and Nicobar Islands
|
32
|
7
|
|
Andhra Pradesh
|
|
2,00,945
|
|
Assam
|
332
|
1,028
|
|
Bihar
|
145
|
22
|
|
Chandigarh
|
3
|
7
|
|
Chhattisgarh
|
1,049
|
43,964
|
|
DNH and DD
|
19
|
4
|
|
Goa
|
|
1
|
|
Gujarat
|
11,355
|
|
|
Haryana
|
14,817
|
719
|
|
Himachal Pradesh
|
12
|
52
|
|
Jammu and Kashmir
|
4
|
729
|
|
Jharkhand
|
30
|
1,495
|
|
Karnataka
|
|
1,82,070
|
|
Kerala
|
18,728
|
1,33,591
|
|
Madhya Pradesh
|
7,891
|
18,352
|
|
Maharashtra
|
1,35,904
|
1,82,991
|
|
Manipur
|
1
|
732
|
|
Meghalaya
|
7,589
|
3,932
|
|
Mizoram
|
|
416
|
|
Nagaland
|
-
|
12
|
|
Puducherry
|
20
|
349
|
|
Punjab
|
13
|
|
|
Rajasthan
|
|
23,761
|
|
Sikkim
|
-
|
32
|
|
Tamil Nadu*
|
18,50,134
|
31,076
|
|
Tripura
|
1
|
54
|
|
Uttar Pradesh
|
1,571
|
1,421
|
|
Uttarakhand
|
2,042
|
2,801
|
|
Total
|
20,51,692
|
8,29,826
|
Note:
* Treatment data is as of 25th October 2021; Testing data is as of 1st week of June 2021
*********
AS
(Release ID: 1778959)
Visitor Counter : 725